 |
PDBsum entry 1d06

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Signaling protein
|
PDB id
|
|

|

|
1d06
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Signaling protein
|
 |
|
Title:
|
 |
Structural basis of dimerization and sensory mechanisms of oxygen- sensing domain of rhizobium meliloti fixl determined at 1.4a resolution
|
|
Structure:
|
 |
Nitrogen fixation regulatory protein fixl. Chain: a. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Sinorhizobium meliloti. Organism_taxid: 382. Expressed in: escherichia coli k12. Expression_system_taxid: 83333.
|
|
Biol. unit:
|
 |
 Dimer (from
)
Dimer (from
)
|
|
Resolution:
|
 |
|
1.40Å
|
R-factor:
|
0.224
|
R-free:
|
0.275
|
|
|
Authors:
|
 |
H.Miyatake,M.Mukai,S.-Y.Park,S.Adachi,K.Tamura,H.Nakamura,K.Nakamura, T.Tsuchiya,T.Iizuka,Y.Shiro
|
Key ref:

|
 |
H.Miyatake
et al.
(2000).
Sensory mechanism of oxygen sensor FixL from Rhizobium meliloti: crystallographic, mutagenesis and resonance Raman spectroscopic studies.
J Mol Biol,
301,
415-431.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
09-Sep-99
|
Release date:
|
15-Mar-00
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P10955
(FIXL_RHIME) -
Sensor protein FixL from Rhizobium meliloti (strain 1021)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
505 a.a.
130 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 6 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.13.3
- histidine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + protein L-histidine = ADP + protein N-phospho-L-histidine
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
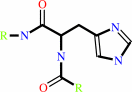 protein L-histidine
protein L-histidine
|
=
|
 ADP
ADP
|
+
|
protein N-phospho-L-histidine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
301:415-431
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
Sensory mechanism of oxygen sensor FixL from Rhizobium meliloti: crystallographic, mutagenesis and resonance Raman spectroscopic studies.
|
|
H.Miyatake,
M.Mukai,
S.Y.Park,
S.Adachi,
K.Tamura,
H.Nakamura,
K.Nakamura,
T.Tsuchiya,
T.Iizuka,
Y.Shiro.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
FixL of Rhizobium meliloti (RmFixL) is a sensor histidine kinase of the
two-component system, which regulates the expression of the genes related to
nitrogen fixation in the root nodule in response to the O(2) levels. The crystal
structure of the sensor domain of FixL (RmFixLH), which contains a heme
(Fe-porphyrin) as a sensing site, was determined at 1.4 A resolution. Based on
the structural and spectroscopic analyses, we propose the O(2) sensing mechanism
that differs from the case proposed in BjFixLH as follows; conformational
changes in the F/G loop, which are induced by steric repulsion between the
bent-bound O(2) and the Ile209 side-chain, would be transmitted to the histidine
kinase domain. Interaction between the iron-bound O(2) and Ile209 was also
observed in the resonance Raman spectra of RmFixLH as evidenced by the fact that
the Fe-O(2) and Fe-CN stretching frequencies were shifted from 575 to 570 cm(-1)
(Fe-O(2)), and 504 to 499 cm(-1), respectively, as the result of the replacement
of Ile209 with an Ala residue. In the I209A mutant of RmFixL, the O(2) sensing
activity was destroyed, thus confirming our proposed mechanism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Dimerization interaction at the N-terminal: (a)
"Leucine-zipper"-like interaction at helix II with CPK
representations. (b) The interaction between helix I and the
RmFixLH core region. The pictures (a) and (b) were depicted with
MOLSCRIPT and RASTER3D. (c) The amino acid sequences of the
leucine zipper-like helices (helix II) of Rhizobium meliloti
(RmFixLH), Bradyrhizobium japonicum (BjFixLH) and Azorhizobium
caulinodans (AcFixLH) are drawn in a-helical wheels, in order to
present the amphipathic nature, in which hydrophobic residues
are colored in yellow, while the others are in light blue.
|
 |
Figure 6.
Figure 6. (a) Heme and its surroundings with distances
(Å) and electron density map (>1s). Distal side residues
(Ile209, Ile210, Leu230, Val232) and proximal residues (His194,
Asn181, Asp195) are shown. (b) Top and side views of a van der
Waals drawing of the structure at the heme distal region.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2000,
301,
415-431)
copyright 2000.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Goblirsch,
R.C.Kurker,
B.R.Streit,
C.M.Wilmot,
and
J.L.DuBois
(2011).
Chlorite dismutases, DyPs, and EfeB: 3 microbial heme enzyme families comprise the CDE structural superfamily.
|
| |
J Mol Biol,
408,
379-398.
|
 |
|
|
|
|
 |
J.D.Satterlee
(2011).
Origins of aging mass loss in recombinant N-terminus and C-terminus deletion mutants of the heme-PAS biosensor domain BjFixLH(140-270).
|
| |
J Inorg Biochem,
105,
609-615.
|
 |
|
|
|
|
 |
J.King-Scott,
P.V.Konarev,
S.Panjikar,
R.Jordanova,
D.I.Svergun,
and
P.A.Tucker
(2011).
Structural characterization of the multidomain regulatory protein Rv1364c from Mycobacterium tuberculosis.
|
| |
Structure,
19,
56-69.
|
 |
|
|
|
|
 |
O.Shoji,
and
Y.Watanabe
(2011).
Design of H2O2-dependent oxidation catalyzed by hemoproteins.
|
| |
Metallomics,
3,
379-388.
|
 |
|
|
|
|
 |
J.Cheung,
and
W.A.Hendrickson
(2010).
Sensor domains of two-component regulatory systems.
|
| |
Curr Opin Microbiol,
13,
116-123.
|
 |
|
|
|
|
 |
A.Möglich,
R.A.Ayers,
and
K.Moffat
(2009).
Structure and signaling mechanism of Per-ARNT-Sim domains.
|
| |
Structure,
17,
1282-1294.
|
 |
|
|
|
|
 |
S.Hennig,
H.M.Strauss,
K.Vanselow,
O.Yildiz,
S.Schulze,
J.Arens,
A.Kramer,
and
E.Wolf
(2009).
Structural and functional analyses of PAS domain interactions of the clock proteins Drosophila PERIOD and mouse PERIOD2.
|
| |
PLoS Biol,
7,
e94.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Yamada,
H.Sugimoto,
M.Kobayashi,
A.Ohno,
H.Nakamura,
and
Y.Shiro
(2009).
Structure of PAS-linked histidine kinase and the response regulator complex.
|
| |
Structure,
17,
1333-1344.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
U.E.Ukaegbu,
and
A.C.Rosenzweig
(2009).
Structure of the redox sensor domain of Methylococcus capsulatus (Bath) MmoS.
|
| |
Biochemistry,
48,
2207-2215.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
B.D.Zoltowski,
and
B.R.Crane
(2008).
Light activation of the LOV protein vivid generates a rapidly exchanging dimer.
|
| |
Biochemistry,
47,
7012-7019.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.J.Watts,
M.S.Johnson,
and
B.L.Taylor
(2008).
Structure-function relationships in the HAMP and proximal signaling domains of the aerotaxis receptor Aer.
|
| |
J Bacteriol,
190,
2118-2127.
|
 |
|
|
|
|
 |
L.M.Podust,
A.Ioanoviciu,
and
P.R.Ortiz de Montellano
(2008).
2.3 A X-ray structure of the heme-bound GAF domain of sensory histidine kinase DosT of Mycobacterium tuberculosis.
|
| |
Biochemistry,
47,
12523-12531.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.A.Ayers,
and
K.Moffat
(2008).
Changes in quaternary structure in the signaling mechanisms of PAS domains.
|
| |
Biochemistry,
47,
12078-12086.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
X.Ma,
N.Sayed,
P.Baskaran,
A.Beuve,
and
F.van den Akker
(2008).
PAS-mediated dimerization of soluble guanylyl cyclase revealed by signal transduction histidine kinase domain crystal structure.
|
| |
J Biol Chem,
283,
1167-1178.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Busch,
J.Lacal,
A.Martos,
J.L.Ramos,
and
T.Krell
(2007).
Bacterial sensor kinase TodS interacts with agonistic and antagonistic signals.
|
| |
Proc Natl Acad Sci U S A,
104,
13774-13779.
|
 |
|
|
|
|
 |
V.Buttani,
A.Losi,
T.Eggert,
U.Krauss,
K.E.Jaeger,
Z.Cao,
and
W.Gärtner
(2007).
Conformational analysis of the blue-light sensing protein YtvA reveals a competitive interface for LOV-LOV dimerization and interdomain interactions.
|
| |
Photochem Photobiol Sci,
6,
41-49.
|
 |
|
|
|
|
 |
K.J.Watts,
K.Sommer,
S.L.Fry,
M.S.Johnson,
and
B.L.Taylor
(2006).
Function of the N-terminal cap of the PAS domain in signaling by the aerotaxis receptor Aer.
|
| |
J Bacteriol,
188,
2154-2162.
|
 |
|
|
|
|
 |
T.Mascher,
J.D.Helmann,
and
G.Unden
(2006).
Stimulus perception in bacterial signal-transducing histidine kinases.
|
| |
Microbiol Mol Biol Rev,
70,
910-938.
|
 |
|
|
|
|
 |
H.Kurokawa,
D.S.Lee,
M.Watanabe,
I.Sagami,
B.Mikami,
C.S.Raman,
and
T.Shimizu
(2004).
A redox-controlled molecular switch revealed by the crystal structure of a bacterial heme PAS sensor.
|
| |
J Biol Chem,
279,
20186-20193.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.H.Hefti,
K.J.Françoijs,
S.C.de Vries,
R.Dixon,
and
J.Vervoort
(2004).
The PAS fold. A redefinition of the PAS domain based upon structural prediction.
|
| |
Eur J Biochem,
271,
1198-1208.
|
 |
|
|
|
|
 |
M.Watanabe,
H.Kurokawa,
T.Yoshimura-Suzuki,
I.Sagami,
and
T.Shimizu
(2004).
Critical roles of Asp40 at the haem proximal side of haem-regulated phosphodiesterase from Escherichia coli in redox potential, auto-oxidation and catalytic control.
|
| |
Eur J Biochem,
271,
3937-3942.
|
 |
|
|
|
|
 |
P.Pellicena,
D.S.Karow,
E.M.Boon,
M.A.Marletta,
and
J.Kuriyan
(2004).
Crystal structure of an oxygen-binding heme domain related to soluble guanylate cyclases.
|
| |
Proc Natl Acad Sci U S A,
101,
12854-12859.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Taguchi,
T.Matsui,
J.Igarashi,
Y.Sasakura,
Y.Araki,
O.Ito,
S.Sugiyama,
I.Sagami,
and
T.Shimizu
(2004).
Binding of oxygen and carbon monoxide to a heme-regulated phosphodiesterase from Escherichia coli. Kinetics and infrared spectra of the full-length wild-type enzyme, isolated PAS domain, and Met-95 mutants.
|
| |
J Biol Chem,
279,
3340-3347.
|
 |
|
|
|
|
 |
L.R.Swem,
B.J.Kraft,
D.L.Swem,
A.T.Setterdahl,
S.Masuda,
D.B.Knaff,
J.M.Zaleski,
and
C.E.Bauer
(2003).
Signal transduction by the global regulator RegB is mediated by a redox-active cysteine.
|
| |
EMBO J,
22,
4699-4708.
|
 |
|
|
|
|
 |
S.Hirata,
T.Matsui,
Y.Sasakura,
S.Sugiyama,
T.Yoshimura,
I.Sagami,
and
T.Shimizu
(2003).
Characterization of Met95 mutants of a heme-regulated phosphodiesterase from Escherichia coli. Optical absorption, magnetic circular dichroism, circular dichroism, and redox potentials.
|
| |
Eur J Biochem,
270,
4771-4779.
|
 |
|
|
|
|
 |
U.Liebl,
L.Bouzhir-Sima,
L.Kiger,
M.C.Marden,
J.C.Lambry,
M.Négrerie,
and
M.H.Vos
(2003).
Ligand binding dynamics to the heme domain of the oxygen sensor Dos from Escherichia coli.
|
| |
Biochemistry,
42,
6527-6535.
|
 |
|
|
|
|
 |
A.Sato,
Y.Sasakura,
S.Sugiyama,
I.Sagami,
T.Shimizu,
Y.Mizutani,
and
T.Kitagawa
(2002).
Stationary and time-resolved resonance Raman spectra of His77 and Met95 mutants of the isolated heme domain of a direct oxygen sensor from Escherichia coli.
|
| |
J Biol Chem,
277,
32650-32658.
|
 |
|
|
|
|
 |
B.Hao,
C.Isaza,
J.Arndt,
M.Soltis,
and
M.K.Chan
(2002).
Structure-based mechanism of O2 sensing and ligand discrimination by the FixL heme domain of Bradyrhizobium japonicum.
|
| |
Biochemistry,
41,
12952-12958.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Park,
C.Suquet,
M.I.Savenkova,
J.D.Satterlee,
and
C.Kang
(2002).
Cloning, purification, crystallization and preliminary X-ray analysis of DOS heme domain, a new heme oxygen sensor in Escherichia coli.
|
| |
Acta Crystallogr D Biol Crystallogr,
58,
1504-1506.
|
 |
|
|
|
|
 |
J.R.Tuckerman,
G.Gonzalez,
E.M.Dioum,
and
M.A.Gilles-Gonzalez
(2002).
Ligand and oxidation-state specific regulation of the heme-based oxygen sensor FixL from Sinorhizobium meliloti.
|
| |
Biochemistry,
41,
6170-6177.
|
 |
|
|
|
|
 |
T.Tomita,
G.Gonzalez,
A.L.Chang,
M.Ikeda-Saito,
and
M.A.Gilles-Gonzalez
(2002).
A comparative resonance Raman analysis of heme-binding PAS domains: heme iron coordination structures of the BjFixL, AxPDEA1, EcDos, and MtDos proteins.
|
| |
Biochemistry,
41,
4819-4826.
|
 |
|
|
|
|
 |
U.Liebl,
L.Bouzhir-Sima,
M.Negrerie,
J.L.Martin,
and
M.H.Vos
(2002).
Ultrafast ligand rebinding in the heme domain of the oxygen sensors FixL and Dos: general regulatory implications for heme-based sensors.
|
| |
Proc Natl Acad Sci U S A,
99,
12771-12776.
|
 |
|
|
|
|
 |
A.L.Chang,
J.R.Tuckerman,
G.Gonzalez,
R.Mayer,
H.Weinhouse,
G.Volman,
D.Amikam,
M.Benziman,
and
M.A.Gilles-Gonzalez
(2001).
Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor.
|
| |
Biochemistry,
40,
3420-3426.
|
 |
|
|
|
|
 |
K.R.Rodgers,
L.Tang,
G.S.Lukat-Rodgers,
and
N.L.Wengenack
(2001).
Insights into the signal transduction mechanism of RmFixL provided by carbon monoxide recombination kinetics.
|
| |
Biochemistry,
40,
12932-12942.
|
 |
|
|
|
|
 |
M.Hefti,
J.Hendle,
C.Enroth,
J.Vervoort,
and
P.A.Tucker
(2001).
Crystallization and preliminary crystallographic data of the PAS domain of the NifL protein from Azotobacter vinelandii.
|
| |
Acta Crystallogr D Biol Crystallogr,
57,
1895-1896.
|
 |
|
|
|
|
 |
M.K.Chan
(2001).
Recent advances in heme-protein sensors.
|
| |
Curr Opin Chem Biol,
5,
216-222.
|
 |
|
|
|
|
 |
S.Crosson,
and
K.Moffat
(2001).
Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction.
|
| |
Proc Natl Acad Sci U S A,
98,
2995-3000.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

