 |
PDBsum entry 1ay5

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.57
- aromatic-amino-acid transaminase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Phenylalanine and Tyrosine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an aromatic L-alpha-amino acid + 2-oxoglutarate = an aromatic oxo-acid + L-glutamate
|
 |
 |
 |
 |
 |
aromatic L-alpha-amino acid
Bound ligand (Het Group name = )
matches with 63.64% similarity
|
+
|
 2-oxoglutarate
2-oxoglutarate
|
=
|
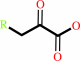 aromatic oxo-acid
aromatic oxo-acid
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
280:443-461
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of Paracoccus denitrificans aromatic amino acid aminotransferase: a substrate recognition site constructed by rearrangement of hydrogen bond network.
|
|
A.Okamoto,
Y.Nakai,
H.Hayashi,
K.Hirotsu,
H.Kagamiyama.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Aminotransferase reversibly catalyzes the transamination reaction by a ping-pong
bi-bi mechanism with pyridoxal 5'-phosphate (PLP) as a cofactor. Various kinds
of aminotransferases developing into catalysts for particular substrates have
been reported. Among the aminotransferases, aromatic amino acid aminotransferase
(EC 2.6.1. 57) catalyzes the transamination reaction with both acidic substrates
and aromatic substrates. To elucidate the multiple substrate recognition
mechanism, we determined the crystal structures of aromatic amino acid
aminotransferase from Paracoccus denitrificans (pdAroAT): unliganded pdAroAT,
pdAroAT in a complex with maleate as an acidic substrate analog, and pdAroAT in
a complex with 3-phenylpropionate as an aromatic substrate analog at 2.33 A, 2.
50 A and 2.30 A resolution, respectively. The pdAroAT molecule is a homo-dimer.
Each subunit has 394 amino acids and one PLP and is divided into small and large
domains. The overall structure of pdAroAT is essentially identical to that of
aspartate aminotransferase (AspAT) which catalyzes the transamination reaction
with only an acidic amino acid. On binding the acidic substrate analog, arginine
292 and 386 form end-on salt bridges with carboxylates of the analog.
Furthermore, binding of the substrate induces the domain movement to close the
active site. The recognition mechanism for the acidic substrate analog in
pdAroAT is identical to that observed in AspAT. Binding of the aromatic
substrate analog causes reorientation of the side-chain of the residues, lysine
16, asparagine 142, arginine 292* and serine 296*, and changes in the position
of water molecules in the active site to form a new hydrogen bond network in
contrast to the active site structure of pdAroAT in the complex with an acidic
substrate analog. Consequently, the rearrangement of the hydrogen bond network
can form recognition sites for both acidic and aromatic side-chains of the
substrate without a conformational change in the backbone structure in pdAroAT.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Ribbon drawing of a molecule of unliganded
pdAroAT viewed along the molecular dyad. α-Helices are colored
red and β-strands yellow. PLP is indicated by the
ball-and-stick model. Only in subunit A, the α-helices are
numbered and N and C-terminals are indicated by N and C,
respectively. Figure produced with MOLSCRIPT [Kraulis 1991].
|
 |
Figure 2.
Figure 2. Active site structures. PLP and inhibitor are
indicated by open bonds and water molecules by the filled
circles. (a) The active site in subunit B of the unliganded
pdAroAT; (b) the active site in subunit B of the maleate complex
of pdAroAT and (c) the active site in subunit B of the
3-phenylpropionate complex of pdAroAT. Produced with the program
ORTEP-II [Johnson 1976].
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(1998,
280,
443-461)
copyright 1998.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Höhne,
S.Schätzle,
H.Jochens,
K.Robins,
and
U.T.Bornscheuer
(2010).
Rational assignment of key motifs for function guides in silico enzyme identification.
|
| |
Nat Chem Biol,
6,
807-813.
|
 |
|
|
|
|
 |
Q.Han,
T.Cai,
D.A.Tagle,
and
J.Li
(2010).
Structure, expression, and function of kynurenine aminotransferases in human and rodent brains.
|
| |
Cell Mol Life Sci,
67,
353-368.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Q.Han,
H.Robinson,
T.Cai,
D.A.Tagle,
and
J.Li
(2009).
Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K.
|
| |
J Med Chem,
52,
2786-2793.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Tomita,
T.Miyagawa,
T.Miyazaki,
S.Fushinobu,
T.Kuzuyama,
and
M.Nishiyama
(2009).
Mechanism for multiple-substrates recognition of alpha-aminoadipate aminotransferase from Thermus thermophilus.
|
| |
Proteins,
75,
348-359.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Marienhagen,
T.Sandalova,
H.Sahm,
L.Eggeling,
and
G.Schneider
(2008).
Insights into the structural basis of substrate recognition by histidinol-phosphate aminotransferase from Corynebacterium glutamicum.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
675-685.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.J.Zelyas,
H.Cai,
T.Kwong,
and
S.E.Jensen
(2008).
Alanylclavam biosynthetic genes are clustered together with one group of clavulanic acid biosynthetic genes in Streptomyces clavuligerus.
|
| |
J Bacteriol,
190,
7957-7965.
|
 |
|
|
|
|
 |
Q.Han,
Y.G.Gao,
H.Robinson,
and
J.Li
(2008).
Structural insight into the mechanism of substrate specificity of aedes kynurenine aminotransferase.
|
| |
Biochemistry,
47,
1622-1630.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.Matsui,
and
K.Harata
(2007).
Implication for buried polar contacts and ion pairs in hyperthermostable enzymes.
|
| |
FEBS J,
274,
4012-4022.
|
 |
|
|
|
|
 |
J.Kim,
D.Kyung,
H.Yun,
B.K.Cho,
J.H.Seo,
M.Cha,
and
B.G.Kim
(2007).
Cloning and characterization of a novel beta-transaminase from Mesorhizobium sp. strain LUK: a new biocatalyst for the synthesis of enantiomerically pure beta-amino acids.
|
| |
Appl Environ Microbiol,
73,
1772-1782.
|
 |
|
|
|
|
 |
B.K.Cho,
J.H.Seo,
T.J.Kang,
J.Kim,
H.Y.Park,
B.S.Lee,
and
B.G.Kim
(2006).
Engineering aromatic L-amino acid transaminase for the asymmetric synthesis of constrained analogs of L-phenylalanine.
|
| |
Biotechnol Bioeng,
94,
842-850.
|
 |
|
|
|
|
 |
Q.Han,
H.Robinson,
Y.G.Gao,
N.Vogelaar,
S.R.Wilson,
M.Rizzi,
and
J.Li
(2006).
Crystal structures of Aedes aegypti alanine glyoxylate aminotransferase.
|
| |
J Biol Chem,
281,
37175-37182.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Hirotsu,
M.Goto,
A.Okamoto,
and
I.Miyahara
(2005).
Dual substrate recognition of aminotransferases.
|
| |
Chem Rec,
5,
160-172.
|
 |
|
|
|
|
 |
A.C.Eliot,
and
J.F.Kirsch
(2004).
Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations.
|
| |
Annu Rev Biochem,
73,
383-415.
|
 |
|
|
|
|
 |
A.Paiardini,
F.Bossa,
and
S.Pascarella
(2004).
Evolutionarily conserved regions and hydrophobic contacts at the superfamily level: The case of the fold-type I, pyridoxal-5'-phosphate-dependent enzymes.
|
| |
Protein Sci,
13,
2992-3005.
|
 |
|
|
|
|
 |
B.Cellini,
M.Bertoldi,
A.Paiardini,
S.D'Aguanno,
and
C.B.Voltattorni
(2004).
Site-directed mutagenesis provides insight into racemization and transamination of alanine catalyzed by Treponema denticola cystalysin.
|
| |
J Biol Chem,
279,
36898-36905.
|
 |
|
|
|
|
 |
B.K.Cho,
H.Y.Park,
J.H.Seo,
K.Kinnera,
B.S.Lee,
and
B.G.Kim
(2004).
Enzymatic resolution for the preparation of enantiomerically enriched D-beta-heterocyclic alanine derivatives using Escherichia coli aromatic L-amino acid transaminase.
|
| |
Biotechnol Bioeng,
88,
512-519.
|
 |
|
|
|
|
 |
S.C.Rothman,
M.Voorhies,
and
J.F.Kirsch
(2004).
Directed evolution relieves product inhibition and confers in vivo function to a rationally designed tyrosine aminotransferase.
|
| |
Protein Sci,
13,
763-772.
|
 |
|
|
|
|
 |
B.K.Cho,
J.H.Seo,
T.W.Kang,
and
B.G.Kim
(2003).
Asymmetric synthesis of L-homophenylalanine by equilibrium-shift using recombinant aromatic L-amino acid transaminase.
|
| |
Biotechnol Bioeng,
83,
226-234.
|
 |
|
|
|
|
 |
V.R.Sobrado,
M.Montemartini-Kalisz,
H.M.Kalisz,
M.C.De La Fuente,
H.J.Hecht,
and
C.Nowicki
(2003).
Involvement of conserved asparagine and arginine residues from the N-terminal region in the catalytic mechanism of rat liver and Trypanosoma cruzi tyrosine aminotransferases.
|
| |
Protein Sci,
12,
1039-1050.
|
 |
|
|
|
|
 |
D.H.Fong,
and
A.M.Berghuis
(2002).
Substrate promiscuity of an aminoglycoside antibiotic resistance enzyme via target mimicry.
|
| |
EMBO J,
21,
2323-2331.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.Nowicki,
G.R.Hunter,
M.Montemartini-Kalisz,
W.Blankenfeldt,
H.Hecht,
and
H.M.Kalisz
(2001).
Recombinant tyrosine aminotransferase from Trypanosoma cruzi: structural characterization and site directed mutagenesis of a broad substrate specificity enzyme.
|
| |
Biochim Biophys Acta,
1546,
268-281.
|
 |
|
|
|
|
 |
K.Soda,
T.Yoshimura,
and
N.Esaki
(2001).
Stereospecificity for the hydrogen transfer of pyridoxal enzyme reactions.
|
| |
Chem Rec,
1,
373-384.
|
 |
|
|
|
|
 |
D.Saadat,
and
D.H.Harrison
(2000).
Mirroring perfection: the structure of methylglyoxal synthase complexed with the competitive inhibitor 2-phosphoglycolate.
|
| |
Biochemistry,
39,
2950-2960.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Schneider,
H.Käck,
and
Y.Lindqvist
(2000).
The manifold of vitamin B6 dependent enzymes.
|
| |
Structure,
8,
R1-R6.
|
 |
|
|
|
|
 |
I.Matsui,
E.Matsui,
Y.Sakai,
H.Kikuchi,
Y.Kawarabayasi,
H.Ura,
S.Kawaguchi,
S.Kuramitsu,
and
K.Harata
(2000).
The molecular structure of hyperthermostable aromatic aminotransferase with novel substrate specificity from Pyrococcus horikoshii.
|
| |
J Biol Chem,
275,
4871-4879.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Okamoto,
S.Ishii,
K.Hirotsu,
and
H.Kagamiyama
(1999).
The active site of Paracoccus denitrificans aromatic amino acid aminotransferase has contrary properties: flexibility and rigidity.
|
| |
Biochemistry,
38,
1176-1184.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.P.Ko,
S.P.Wu,
W.Z.Yang,
H.Tsai,
and
H.S.Yuan
(1999).
Crystallization and preliminary crystallographic analysis of the Escherichia coli tyrosine aminotransferase.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
1474-1477.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Blankenfeldt,
C.Nowicki,
M.Montemartini-Kalisz,
H.M.Kalisz,
and
H.J.Hecht
(1999).
Crystal structure of Trypanosoma cruzi tyrosine aminotransferase: substrate specificity is influenced by cofactor binding mode.
|
| |
Protein Sci,
8,
2406-2417.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.N.Jansonius
(1998).
Structure, evolution and action of vitamin B6-dependent enzymes.
|
| |
Curr Opin Struct Biol,
8,
759-769.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

