 |
PDBsum entry 6p7c
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
D104a/s128a s. Typhimurium siroheme synthase
|
|
Structure:
|
 |
Siroheme synthase. Chain: a, b. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Salmonella typhimurium. Organism_taxid: 90371. Gene: cysg, coba, aap89_23165, abo94_22995, af480_23265, af488_22710, af489_21700, aic76_23675, axr84_23260, axu58_22185, c2253_19735, cd48_22680, ce87_23070, cet98_25025, cvr97_13625, d7f20_23535, d7h43_21790, dj388_17225, dm376_19045, e2f01_22980, fcj89_03505, fg594_22565, fg605_22440, fg608_22845, fg609_22120, fg610_23420, fg612_22925, fg614_17700, fg736_21980, fjv50_24115, fjv51_21965, fjv53_21305, fjv54_21440, fjv55_23340, fjv56_23845,
|
|
Resolution:
|
 |
|
2.76Å
|
R-factor:
|
0.193
|
R-free:
|
0.285
|
|
|
Authors:
|
 |
J.M.Pennington,M.E.Stroupe
|
|
Key ref:
|
 |
J.M.Pennington
et al.
(2020).
Siroheme synthase orients substrates for dehydrogenase and chelatase activities in a common active site.
Nat Commun,
11,
864.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
05-Jun-19
|
Release date:
|
26-Feb-20
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P25924
(CYSG_SALTY) -
Siroheme synthase from Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
457 a.a.
451 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.1.3.1.76
- precorrin-2 dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Corrin and Siroheme Biosynthesis (part 2)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
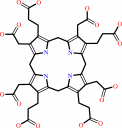
precorrin-2 + NAD+ = sirohydrochlorin + NADH + 2 H+
|
 |
 |
 |
 |
 |
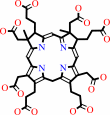 precorrin-2
precorrin-2
|
+
|
 NAD(+)
NAD(+)
|
=
|
 sirohydrochlorin
sirohydrochlorin
|
+
|
 NADH
NADH
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.1.1.107
- uroporphyrinogen-III C-methyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
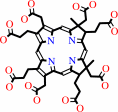
uroporphyrinogen III + 2 S-adenosyl-L-methionine = precorrin-2 + 2 S-adenosyl-L-homocysteine + H+
|
 |
 |
 |
 |
 |
 uroporphyrinogen III
uroporphyrinogen III
|
+
|
 2
×
S-adenosyl-L-methionine
2
×
S-adenosyl-L-methionine
|
=
|
 precorrin-2
precorrin-2
|
+
|
 2
×
S-adenosyl-L-homocysteine
2
×
S-adenosyl-L-homocysteine
|
+
|
2
×
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.4.99.1.4
- sirohydrochlorin ferrochelatase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
siroheme + 2 H+ = sirohydrochlorin + Fe2+
|
 |
 |
 |
 |
 |
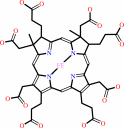 siroheme
siroheme
|
+
|
2
×
H(+)
|
=
|
 sirohydrochlorin
sirohydrochlorin
|
+
|
2
×
Fe(2+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Commun
11:864
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
Siroheme synthase orients substrates for dehydrogenase and chelatase activities in a common active site.
|
|
J.M.Pennington,
M.Kemp,
L.McGarry,
Y.Chen,
M.E.Stroupe.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Siroheme is the central cofactor in a conserved class of sulfite and nitrite
reductases that catalyze the six-electron reduction of sulfite to sulfide and
nitrite to ammonia. In Salmonella enterica serovar Typhimurium, siroheme is
produced by a trifunctional enzyme, siroheme synthase (CysG). A bifunctional
active site that is distinct from its methyltransferase activity catalyzes the
final two steps, NAD+-dependent dehydrogenation and iron chelation.
How this active site performs such different chemistries is unknown. Here, we
report the structures of CysG bound to precorrin-2, the initial substrate;
sirohydrochlorin, the dehydrogenation product/chelation substrate; and a
cobalt-sirohydrochlorin product. We identified binding poses for all three
tetrapyrroles and tested the roles of specific amino acids in both activities to
give insights into how a bifunctional active site catalyzes two different
chemistries and acts as an iron-specific chelatase in the final step of siroheme
synthesis.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

