 |
PDBsum entry 5ctc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/transferase inhibitor
|
PDB id
|
|

|

|
5ctc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase/transferase inhibitor
|
 |
|
Title:
|
 |
Humanized yeast acc carboxyltransferase domain bound to tert-butyl 7- [(7-methyl-1h-indazol-5-yl)carbonyl]-2,7-diazaspiro[3.5]nonane-2- carboxylate
|
|
Structure:
|
 |
Acetyl-coa carboxylase. Chain: a, b, c. Fragment: carboxyltransferase domain (unp residues 1476-2233). Synonym: acc, fatty acid synthetase 3, mRNA transport-defective protein 7. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Saccharomyces cerevisiae. Baker's yeast. Organism_taxid: 4932. Gene: acc1, abp2, fas3, mtr7, ynr016c, n3175. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.70Å
|
R-factor:
|
0.176
|
R-free:
|
0.201
|
|
|
Authors:
|
 |
F.F.Vajdos
|
|
Key ref:
|
 |
D.W.Kung
et al.
(2015).
Discovery of spirocyclic-diamine inhibitors of mammalian acetyl CoA-carboxylase.
Bioorg Med Chem Lett,
25,
5352-5356.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
23-Jul-15
|
Release date:
|
14-Oct-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q00955
(ACAC_YEAST) -
Acetyl-CoA carboxylase from Saccharomyces cerevisiae (strain ATCC 204508 / S288c)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
2233 a.a.
691 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 9 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.6.3.4.14
- biotin carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N6-biotinyl-L-lysyl-[protein] + hydrogencarbonate + ATP = N6- carboxybiotinyl-L-lysyl-[protein] + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
N(6)-biotinyl-L-lysyl-[protein]
|
+
|
 hydrogencarbonate
hydrogencarbonate
|
+
|
 ATP
ATP
|
=
|
N(6)- carboxybiotinyl-L-lysyl-[protein]
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.6.4.1.2
- acetyl-CoA carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydrogencarbonate + acetyl-CoA + ATP = malonyl-CoA + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 hydrogencarbonate
hydrogencarbonate
|
+
|
 acetyl-CoA
acetyl-CoA
|
+
|
 ATP
ATP
|
=
|
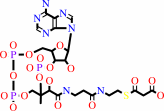 malonyl-CoA
malonyl-CoA
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Biotin
|
 |
 |
 |
 |
 |
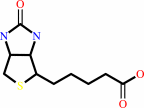 Biotin
Biotin
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Bioorg Med Chem Lett
25:5352-5356
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
Discovery of spirocyclic-diamine inhibitors of mammalian acetyl CoA-carboxylase.
|
|
D.W.Kung,
D.A.Griffith,
W.P.Esler,
F.F.Vajdos,
A.M.Mathiowetz,
S.D.Doran,
P.A.Amor,
S.W.Bagley,
T.Banks,
S.Cabral,
K.Ford,
C.N.Garcia-Irizarry,
M.S.Landis,
K.Loomis,
K.McPherson,
M.Niosi,
K.L.Rockwell,
C.Rose,
A.C.Smith,
J.A.Southers,
S.Tapley,
M.Tu,
J.J.Valentine.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A novel series of spirocyclic-diamine based, isoform non-selective inhibitors of
acetyl-CoA carboxylase (ACC) is described. These spirodiamine derivatives were
discovered by design of a library to mimic the structural rigidity and
hydrogen-bonding pattern observed in the co-crystal structure of spirochromanone
inhibitor I. The lead compound 3.5.1 inhibited de novo lipogenesis in rat
hepatocytes, with an IC50 of 0.30μM.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

