 |
PDBsum entry 4eeg
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of human m340h-beta-1,4-galactosyltransferase-1 (m340h-b4gal-t1) in complex with glcnac-beta1,6-gal-beta
|
|
Structure:
|
 |
Beta-1,4-galactosyltransferase 1. Chain: a, b, c. Fragment: catalytic domain. Synonym: beta-1,4-galtase 1, beta4gal-t1, b4gal-t1, udp-gal:beta- glcnac beta-1,4-galactosyltransferase 1, udp-galactose:beta-n- acetylglucosamine beta-1,4-galactosyltransferase 1, lactose synthase a protein, n-acetyllactosamine synthase, nal synthase, beta-n- acetylglucosaminylglycopeptide beta-1,4-galactosyltransferase, beta- n-acetylglucosaminyl-glycolipid beta-1,4-galactosyltransferase,
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: b4galt1, ggtb2. Expressed in: escherichia coli. Expression_system_taxid: 511693.
|
|
Resolution:
|
 |
|
2.20Å
|
R-factor:
|
0.202
|
R-free:
|
0.244
|
|
|
Authors:
|
 |
B.Ramakrishnan,P.K.Qasba
|
|
Key ref:
|
 |
B.Ramakrishnan
et al.
(2012).
Binding of N-acetylglucosamine (GlcNAc) β1-6-branched oligosaccharide acceptors to β4-galactosyltransferase I reveals a new ligand binding mode.
J Biol Chem,
287,
28666-28674.
PubMed id:
|
 |
|
Date:
|
 |
|
28-Mar-12
|
Release date:
|
04-Jul-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P15291
(B4GT1_HUMAN) -
Beta-1,4-galactosyltransferase 1 from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
398 a.a.
273 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 3 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.4.1.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.4.1.22
- lactose synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-glucose + UDP-alpha-D-galactose = lactose + UDP + H+
|
 |
 |
 |
 |
 |
D-glucose
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
UDP-alpha-D-galactose
|
=
|
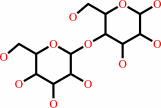 lactose
lactose
|
+
|
 UDP
UDP
|
+
|
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.4.1.275
- neolactotriaosylceramide beta-1,4-galactosyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a beta-D-GlcNAc-(1->3)-beta-D-Gal-(1->4)-beta-D-Glc-(1<->1)- Cer(d18:1(4E)) + UDP-alpha-D-galactose = a neolactoside nLc4Cer(d18:1(4E)) + UDP + H+
|
 |
 |
 |
 |
 |
beta-D-GlcNAc-(1->3)-beta-D-Gal-(1->4)-beta-D-Glc-(1<->1)- Cer(d18:1(4E))
|
+
|
UDP-alpha-D-galactose
|
=
|
neolactoside nLc4Cer(d18:1(4E))
|
+
|
 UDP
UDP
|
+
|
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.2.4.1.38
- beta-N-acetylglucosaminylglycopeptide beta-1,4-galactosyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an N-acetyl-beta-D-glucosaminyl derivative + UDP-alpha-D-galactose = a beta-D-galactosyl-(1->4)-N-acetyl-beta-D-glucosaminyl derivative + UDP + H+
|
 |
 |
 |
 |
 |
N-acetyl-beta-D-glucosaminyl derivative
|
+
|
UDP-alpha-D-galactose
|
=
|
beta-D-galactosyl-(1->4)-N-acetyl-beta-D-glucosaminyl derivative
|
+
|
 UDP
UDP
|
+
|
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.2.4.1.90
- N-acetyllactosamine synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-acetyl-D-glucosamine + UDP-alpha-D-galactose = beta-D-galactosyl- (1->4)-N-acetyl-D-glucosamine + UDP + H+
|
 |
 |
 |
 |
 |
N-acetyl-D-glucosamine
Bound ligand (Het Group name = )
matches with 93.33% similarity
|
+
|
UDP-alpha-D-galactose
|
=
|
beta-D-galactosyl- (1->4)-N-acetyl-D-glucosamine
|
+
|
 UDP
UDP
|
+
|
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Chem
287:28666-28674
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Binding of N-acetylglucosamine (GlcNAc) β1-6-branched oligosaccharide acceptors to β4-galactosyltransferase I reveals a new ligand binding mode.
|
|
B.Ramakrishnan,
E.Boeggeman,
P.K.Qasba.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
N-acetyllactosamine is the most prevalent disaccharide moiety in the glycans on
the surface of mammalian cells and often found as repeat units in the linear and
branched polylactosamines, known as i- and I-antigen, respectively. The
β1-4-galactosyltransferase-I (β4Gal-T1) enzyme is responsible for the
synthesis of the N-acetyllactosamine moiety. To understand its oligosaccharide
acceptor specificity, we have previously investigated the binding of tri- and
pentasaccharides of N-glycan with a GlcNAc at their nonreducing end and found
that the extended sugar moiety in these acceptor substrates binds to the crevice
present at the acceptor substrate binding site of the β4Gal-T1 molecule. Here
we report seven crystal structures of β4Gal-T1 in complex with an
oligosaccharide acceptor with a nonreducing end GlcNAc that has a
β1-6-glycosidic link and that are analogous to either N-glycan or i/I-antigen.
In the crystal structure of the complex of β4Gal-T1 with I-antigen analog
pentasaccharide, the β1-6-branched GlcNAc moiety is bound to the sugar acceptor
binding site of the β4Gal-T1 molecule in a way similar to the crystal
structures described previously; however, the extended linear tetrasaccharide
moiety does not interact with the previously found extended sugar binding site
on the β4Gal-T1 molecule. Instead, it interacts with the different hydrophobic
surface of the protein molecule formed by the residues Tyr-276, Trp-310, and
Phe-356. Results from the present and previous studies suggest that β4Gal-T1
molecule has two different oligosaccharide binding regions for the binding of
the extended oligosaccharide moiety of the acceptor substrate.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

