 |
PDBsum entry 4d5e
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of recombinant wildtype cdh
|
|
Structure:
|
 |
Cyclohexane-1,2-dione hydrolase. Chain: a, b. Engineered: yes
|
|
Source:
|
 |
Azoarcus sp. Bh72. Organism_taxid: 62928. Strain: bh72. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
1.43Å
|
R-factor:
|
0.123
|
R-free:
|
0.147
|
|
|
Authors:
|
 |
S.Loschonsky,T.Wacker,S.Waltzer,P.P.Giovannini,M.J.Mcleish, S.L.A.Andrade,M.Mueller
|
|
Key ref:
|
 |
S.Loschonsky
et al.
(2014).
Extended reaction scope of thiamine diphosphate dependent cyclohexane-1,2-dione hydrolase: from C-C bond cleavage to C-C bond ligation.
Angew Chem Int Ed Engl,
53,
14402-14406.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
03-Nov-14
|
Release date:
|
21-Jan-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0CH62
(CHDH_AZOSP) -
Cyclohexane-1,2-dione hydrolase from Azoarcus sp
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
589 a.a.
586 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.7.1.11
- cyclohexane-1,2-dione hydrolase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
cyclohexan-1,2-dione + H2O = 6-oxohexanoate + H+
|
 |
 |
 |
 |
 |
cyclohexan-1,2-dione
|
+
|
H2O
|
=
|
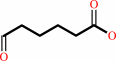 6-oxohexanoate
6-oxohexanoate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Angew Chem Int Ed Engl
53:14402-14406
(2014)
|
|
PubMed id:
|
|
|
|

|
| |
|
Extended reaction scope of thiamine diphosphate dependent cyclohexane-1,2-dione hydrolase: from C-C bond cleavage to C-C bond ligation.
|
|
S.Loschonsky,
T.Wacker,
S.Waltzer,
P.P.Giovannini,
M.J.McLeish,
S.L.Andrade,
M.Müller.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
ThDP-dependent cyclohexane-1,2-dione hydrolase (CDH) catalyzes the CC bond
cleavage of cyclohexane-1,2-dione to 6-oxohexanoate, and the asymmetric benzoin
condensation between benzaldehyde and pyruvate. One of the two reactivities of
CDH was selectively knocked down by mutation experiments. CDH-H28A is much less
able to catalyze the CC bond formation, while the ability for CC bond
cleavage is still intact. The double variant CDH-H28A/N484A shows the opposite
behavior and catalyzes the addition of pyruvate to cyclohexane-1,2-dione,
resulting in the formation of a tertiary alcohol. Several acyloins of tertiary
alcohols are formed with 54-94 % enantiomeric excess. In addition to pyruvate,
methyl pyruvate and butane-2,3-dione are alternative donor substrates for CC
bond formation. Thus, the very rare aldehyde-ketone cross-benzoin reaction has
been solved by design of an enzyme variant.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

