 |
PDBsum entry 3faq

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
3faq
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.7
- peroxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 a phenolic donor + H2O2 = 2 a phenolic radical donor + 2 H2O
|
 |
 |
 |
 |
 |
2
×
a phenolic donor
|
+
|
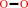 H2O2
H2O2
|
=
|
2
×
a phenolic radical donor
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Heme
|
 |
 |
 |
 |
 |
Heme
Bound ligand (Het Group name =
HEM)
matches with 95.45% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
284:14849-14856
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural evidence of substrate specificity in mammalian peroxidases: Structure of the thiocyanate complex with lactoperoxidase and its interactions at 2.4 angstrom resolution.
|
|
I.A.Sheikh,
A.K.Singh,
N.Singh,
M.Sinha,
S.B.Singh,
A.Bhushan,
P.Kaur,
A.Srinivasan,
S.Sharma,
T.P.Singh.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of the complex of lactoperoxidase (LPO) with its
physiological substrate thiocyanate (SCN-) has been determined at 2.4A
resolution. It revealed that the SCN- ion is bound to LPO in the distal heme
cavity. The observed orientation of SCN- ion shows that the sulfur atom is
closer to the heme iron than the nitrogen atom. The nitrogen atom of SCN- forms
a hydrogen bond with water molecule W6'. This water molecule is stabilized by
two hydrogen bonds with Gln423 NE2 and Phe422 O. In contrast, the placement of
SCN- ion in the structure of myeloperoxidase (MPO) occurs with an opposite
orientation in which the nitrogen atom is closer to the heme iron than the
sulfur atom. The site corresponding to the positions of Gln423, Phe422 O and W6'
in LPO is occupied primarily by the side chain of Phe407 in MPO due to an
entirely different conformation of the loop corresponding to the segment,
Arg418-Phe431 of LPO. This arrangement in MPO does not favour a similar
orientation of SCN- ion. The orientation of the catalytic product OSCN- ion as
reported in the structure of LPO-OSCN- is similar to the orientation of SCN- in
the structure of LPO-SCN-. Similarly, in the structure of LPO-SCN--CN- in which
CN- binds at the position of W1, the position and orientation of SCN- ion are
also identical to that observed in the structure of LPO-SCN-.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Difference Fourier |F[o] – F[c]| map indicating the
presence of SCN^– ions in the complex of LPO·SCN^–.
The cutoff on the left of the pearshaped electron density
corresponds to 2σ (blue), and on the right, it is at 9σ (red).
|
 |
Figure 2.
Difference Fourier map calculated in the structures of
LPO·SCN^–·CN^– for SCN^– and CN^– ions.
The electron density from the difference Fourier map with 2σ
cutoff on the left and 9σ cutoff on the right. The rod-like
density for the CN^– ion is also observed at the distal heme
side.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2009,
284,
14849-14856)
copyright 2009.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.Banerjee,
P.G.Furtmüller,
and
C.Obinger
(2011).
Bovine lactoperoxidase - a versatile one- and two-electron catalyst of high structural and thermal stability.
|
| |
Biotechnol J,
6,
231-243.
|
 |
|
|
|
|
 |
A.K.Singh,
N.Singh,
A.Tiwari,
M.Sinha,
G.S.Kushwaha,
P.Kaur,
A.Srinivasan,
S.Sharma,
and
T.P.Singh
(2010).
First structural evidence for the mode of diffusion of aromatic ligands and ligand-induced closure of the hydrophobic channel in heme peroxidases.
|
| |
J Biol Inorg Chem,
15,
1099-1107.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.K.Singh,
R.P.Kumar,
N.Pandey,
N.Singh,
M.Sinha,
A.Bhushan,
P.Kaur,
S.Sharma,
and
T.P.Singh
(2010).
Mode of binding of the tuberculosis prodrug isoniazid to heme peroxidases: binding studies and crystal structure of bovine lactoperoxidase with isoniazid at 2.7 A resolution.
|
| |
J Biol Chem,
285,
1569-1576.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.K.Singh,
N.Singh,
M.Sinha,
A.Bhushan,
P.Kaur,
A.Srinivasan,
S.Sharma,
and
T.P.Singh
(2009).
Binding modes of aromatic ligands to mammalian heme peroxidases with associated functional implications: crystal structures of lactoperoxidase complexes with acetylsalicylic acid, salicylhydroxamic acid, and benzylhydroxamic acid.
|
| |
J Biol Chem,
284,
20311-20318.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

