 |
PDBsum entry 2x86

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Isomerase
|
 |
|
Title:
|
 |
Agme bound to adp-b-mannose
|
|
Structure:
|
 |
Adp-l-glycero-d-manno-heptose-6-epimerase. Chain: a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r, s, t. Synonym: agme, adp-hep 6-epimerase, adp-l-glycero-beta-d-manno- heptose-6-epimerase, adp-glyceromanno-heptose 6-epimerase. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.80Å
|
R-factor:
|
0.253
|
R-free:
|
0.272
|
|
|
Authors:
|
 |
T.Kowatz,J.P.Morrison,M.E.Tanner,J.H.Naismith
|
|
Key ref:
|
 |
T.Kowatz
et al.
(2010).
The crystal structure of the Y140F mutant of ADP-L-glycero-D-manno-heptose 6-epimerase bound to ADP-beta-D-mannose suggests a one base mechanism.
Protein Sci,
19,
1337-1343.
PubMed id:
|
 |
|
Date:
|
 |
|
06-Mar-10
|
Release date:
|
16-Mar-10
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P67911
(HLDD_ECO57) -
ADP-L-glycero-D-manno-heptose-6-epimerase from Escherichia coli O157:H7
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
310 a.a.
307 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.1.3.20
- ADP-glyceromanno-heptose 6-epimerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ADP-D-glycero-beta-D-manno-heptose = ADP-L-glycero-beta-D-manno-heptose
|
 |
 |
 |
 |
 |
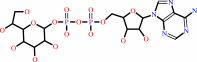 ADP-D-glycero-D-manno-heptose
ADP-D-glycero-D-manno-heptose
|
=
|
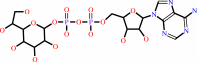 ADP-L-glycero-D-manno-heptose
ADP-L-glycero-D-manno-heptose
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
NAD(+)
|
 |
 |
 |
 |
 |
NAD(+)
Bound ligand (Het Group name =
NAP)
matches with 91.67% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Protein Sci
19:1337-1343
(2010)
|
|
PubMed id:
|
|
|
|

|
| |
|
The crystal structure of the Y140F mutant of ADP-L-glycero-D-manno-heptose 6-epimerase bound to ADP-beta-D-mannose suggests a one base mechanism.
|
|
T.Kowatz,
J.P.Morrison,
M.E.Tanner,
J.H.Naismith.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Bacteria synthesize a wide array of unusual carbohydrate molecules, which they
use in a variety of ways. The carbohydrate L-glycero-D-manno-heptose is an
important component of lipopolysaccharide and is synthesized in a complex series
of enzymatic steps. One step involves the epimerization at the C6'' position
converting ADP-D-glycero-D-manno-heptose into ADP-L-glycero-D-manno-heptose. The
enzyme responsible is a member of the short chain dehydrogenase superfamily,
known as ADP-L-glycero-D-manno-heptose 6-epimerase (AGME). The structure of the
enzyme was known but the arrangement of the catalytic site with respect to the
substrate is unclear. We now report the structure of AGME bound to a substrate
mimic, ADP-beta-D-mannose, which has the same stereochemical configuration as
the substrate. The complex identifies the key residues and allows mechanistic
insight into this novel enzyme.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

