 |
PDBsum entry 2hcs

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.1.1.56
- mRNA (guanine-N(7))-methyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L- methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-homocysteine
|
 |
 |
 |
 |
 |
5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 S-adenosyl-L- methionine
S-adenosyl-L- methionine
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
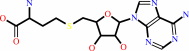 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.1.1.57
- methyltransferase cap1.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA + S-adenosyl-L-homocysteine + H+
|
 |
 |
 |
 |
 |
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA
|
+
|
 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.7.7.48
- RNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
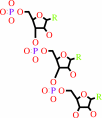 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.3.4.21.91
- flavivirin.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Selective hydrolysis of Xaa-Xaa-|-Xbb bonds in which each of the Xaa can be either Arg or Lys and Xbb can be either Ser or Ala.
|
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.3.6.1.15
- nucleoside-triphosphate phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-triphosphate + H2O = a ribonucleoside 5'-diphosphate + phosphate + H+
|
 |
 |
 |
 |
 |
ribonucleoside 5'-triphosphate
|
+
|
H2O
|
=
|
ribonucleoside 5'-diphosphate
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 6:
|
 |
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
282:10678-10689
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5.
|
|
H.Malet,
M.P.Egloff,
B.Selisko,
R.E.Butcher,
P.J.Wright,
M.Roberts,
A.Gruez,
G.Sulzenbacher,
C.Vonrhein,
G.Bricogne,
J.M.Mackenzie,
A.A.Khromykh,
A.D.Davidson,
B.Canard.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Viruses of the family Flaviviridae are important human and animal pathogens.
Among them, the Flaviviruses dengue (DENV) and West Nile (WNV) cause regular
outbreaks with fatal outcomes. The RNA-dependent RNA polymerase (RdRp) activity
of the non-structural protein 5 (NS5) is a key activity for viral RNA
replication. In this study, crystal structures of enzymatically active and
inactive WNV RdRp domains were determined at 3.0- and 2.35-A resolution,
respectively. The determined structures were shown to be mostly similar to the
RdRps of the Flaviviridae members hepatitis C and bovine viral diarrhea virus,
although with unique elements characteristic for the WNV RdRp. Using a reverse
genetic system, residues involved in putative interactions between the RNA-cap
methyltransferase (MTase) and the RdRp domain of Flavivirus NS5 were identified.
This allowed us to propose a model for the structure of the full-length WNV NS5
by in silico docking of the WNV MTase domain (modeled from our previously
determined structure of the DENV MTase domain) onto the RdRp domain. The
Flavivirus RdRp domain structure determined here should facilitate both the
design of anti-Flavivirus drugs and structure-function studies of the Flavivirus
replication complex in which the multifunctional NS5 protein plays a central
role.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Crystal structure of WNV POL1 and comparison with
HCV RdRp. A, stereo view of a ribbon representation of WNV POL1
in its front orientation. The palm, thumb, and fingers domains
and the priming loop are colored in green, red, dark blue, and
purple, respectively.  -Helices and -Helices and  -sheets
are indicated. Insertions in WNV POL1 compared with HCV RdRp are
displayed in yellow, and major structural differences are shown
in orange. These and other figures were prepared with PyMOL. B,
ribbon representation of HCV RdRp in its front orientation (50)
(PDB code 1NB6). The color code is the same as in A. Insertions
in HCV RdRp compared with WNV are colored in yellow. -sheets
are indicated. Insertions in WNV POL1 compared with HCV RdRp are
displayed in yellow, and major structural differences are shown
in orange. These and other figures were prepared with PyMOL. B,
ribbon representation of HCV RdRp in its front orientation (50)
(PDB code 1NB6). The color code is the same as in A. Insertions
in HCV RdRp compared with WNV are colored in yellow.
|
 |
Figure 3.
FIGURE 3. Divalent ion binding site in WNV POL. Stereo view
of the calcium/magnesium ion non-catalytic binding site. The
POL2 model is represented in green sticks and the corresponding
electronic density in blue. The Ca^2+ ion is shown as a green
sphere. The figure is centered on the aspartic acids of motifs A
and C colored in yellow. Coordination with Asp^536 (motif A) and
Asp^669 (motif C) are indicated by black dotted lines.
Corresponding aspartic acids of motifs A and C in Phi6 (Asp^324,
Asp^453, and Asp^454) (PDB code 1HI0) are represented in magenta
sticks. The position of the two ions in the catalytic position,
inferred from the Phi6 RdRp structure, is indicated in purple.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2007,
282,
10678-10689)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Z.Jin,
J.Deval,
K.A.Johnson,
and
D.C.Swinney
(2011).
Characterization of the elongation complex of dengue virus RNA polymerase: assembly, kinetics of nucleotide incorporation, and fidelity.
|
| |
J Biol Chem,
286,
2067-2077.
|
 |
|
|
|
|
 |
L.J.Yap,
D.Luo,
K.Y.Chung,
S.P.Lim,
C.Bodenreider,
C.Noble,
P.Y.Shi,
and
J.Lescar
(2010).
Crystal structure of the dengue virus methyltransferase bound to a 5'-capped octameric RNA.
|
| |
PLoS One,
5,
0.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Laurent-Rolle,
E.F.Boer,
K.J.Lubick,
J.B.Wolfinbarger,
A.B.Carmody,
B.Rockx,
W.Liu,
J.Ashour,
W.L.Shupert,
M.R.Holbrook,
A.D.Barrett,
P.W.Mason,
M.E.Bloom,
A.García-Sastre,
A.A.Khromykh,
and
S.M.Best
(2010).
The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling.
|
| |
J Virol,
84,
3503-3515.
|
 |
|
|
|
|
 |
P.Niyomrattanakit,
Y.L.Chen,
H.Dong,
Z.Yin,
M.Qing,
J.F.Glickman,
K.Lin,
D.Mueller,
H.Voshol,
J.Y.Lim,
S.Nilar,
T.H.Keller,
and
P.Y.Shi
(2010).
Inhibition of dengue virus polymerase by blocking of the RNA tunnel.
|
| |
J Virol,
84,
5678-5686.
|
 |
|
|
|
|
 |
A.Sampath,
and
R.Padmanabhan
(2009).
Molecular targets for flavivirus drug discovery.
|
| |
Antiviral Res,
81,
6.
|
 |
|
|
|
|
 |
B.E.Pickett,
and
E.J.Lefkowitz
(2009).
Recombination in West Nile Virus: minimal contribution to genomic diversity.
|
| |
Virol J,
6,
165.
|
 |
|
|
|
|
 |
B.J.Geiss,
H.Stahla,
A.M.Hannah,
H.H.Gari,
and
S.M.Keenan
(2009).
Focus on flaviviruses: current and future drug targets.
|
| |
Future Med Chem,
1,
327.
|
 |
|
|
|
|
 |
D.Ghosh,
and
A.Basu
(2009).
Japanese encephalitis-a pathological and clinical perspective.
|
| |
PLoS Negl Trop Dis,
3,
e437.
|
 |
|
|
|
|
 |
K.Ellencrona,
A.Syed,
and
M.Johansson
(2009).
Flavivirus NS5 associates with host-cell proteins zonula occludens-1 (ZO-1) and regulating synaptic membrane exocytosis-2 (RIMS2) via an internal PDZ binding mechanism.
|
| |
Biol Chem,
390,
319-323.
|
 |
|
|
|
|
 |
M.Issur,
B.J.Geiss,
I.Bougie,
F.Picard-Jean,
S.Despins,
J.Mayette,
S.E.Hobdey,
and
M.Bisaillon
(2009).
The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure.
|
| |
RNA,
15,
2340-2350.
|
 |
|
|
|
|
 |
M.S.Diamond
(2009).
Mechanisms of evasion of the type I interferon antiviral response by flaviviruses.
|
| |
J Interferon Cytokine Res,
29,
521-530.
|
 |
|
|
|
|
 |
Q.Y.Koo,
A.M.Khan,
K.O.Jung,
S.Ramdas,
O.Miotto,
T.W.Tan,
V.Brusic,
J.Salmon,
and
J.T.August
(2009).
Conservation and variability of West Nile virus proteins.
|
| |
PLoS ONE,
4,
e5352.
|
 |
|
|
|
|
 |
R.A.Hall,
S.E.Tan,
B.Selisko,
R.Slade,
J.Hobson-Peters,
B.Canard,
M.Hughes,
J.Y.Leung,
E.Balmori-Melian,
S.Hall-Mendelin,
K.B.Pham,
D.C.Clark,
N.A.Prow,
and
A.A.Khromykh
(2009).
Monoclonal antibodies to the West Nile virus NS5 protein map to linear and conformational epitopes in the methyltransferase and polymerase domains.
|
| |
J Gen Virol,
90,
2912-2922.
|
 |
|
|
|
|
 |
A.Gruez,
B.Selisko,
M.Roberts,
G.Bricogne,
C.Bussetta,
I.Jabafi,
B.Coutard,
A.M.De Palma,
J.Neyts,
and
B.Canard
(2008).
The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases.
|
| |
J Virol,
82,
9577-9590.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.Zhang,
H.Dong,
Y.Zhou,
and
P.Y.Shi
(2008).
Genetic interactions among the West Nile virus methyltransferase, the RNA-dependent RNA polymerase, and the 5' stem-loop of genomic RNA.
|
| |
J Virol,
82,
7047-7058.
|
 |
|
|
|
|
 |
C.E.Gardella-Garcia,
G.Perez-Ramirez,
J.Navarrete-Espinosa,
A.Cisneros,
F.Jimenez-Rojas,
L.R.Ramírez-Palacios,
R.Rosado-Leon,
M.Camacho-Nuez,
and
M.d.e. .L.Munoz
(2008).
Specific genetic markers for detecting subtypes of dengue virus serotype-2 in isolates from the states of Oaxaca and Veracruz, Mexico.
|
| |
BMC Microbiol,
8,
117.
|
 |
|
|
|
|
 |
D.Ghosh,
and
A.Basu
(2008).
Present perspectives on flaviviral chemotherapy.
|
| |
Drug Discov Today,
13,
619-624.
|
 |
|
|
|
|
 |
D.Luo,
T.Xu,
C.Hunke,
G.Grüber,
S.G.Vasudevan,
and
J.Lescar
(2008).
Crystal structure of the NS3 protease-helicase from dengue virus.
|
| |
J Virol,
82,
173-183.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Werme,
M.Wigerius,
and
M.Johansson
(2008).
Tick-borne encephalitis virus NS5 associates with membrane protein scribble and impairs interferon-stimulated JAK-STAT signalling.
|
| |
Cell Microbiol,
10,
696-712.
|
 |
|
|
|
|
 |
M.Hass,
M.Lelke,
C.Busch,
B.Becker-Ziaja,
and
S.Günther
(2008).
Mutational evidence for a structural model of the Lassa virus RNA polymerase domain and identification of two residues, Gly1394 and Asp1395, that are critical for transcription but not replication of the genome.
|
| |
J Virol,
82,
10207-10217.
|
 |
|
|
|
|
 |
M.M.Poranen,
P.S.Salgado,
M.R.Koivunen,
S.Wright,
D.H.Bamford,
D.I.Stuart,
and
J.M.Grimes
(2008).
Structural explanation for the role of Mn2+ in the activity of phi6 RNA-dependent RNA polymerase.
|
| |
Nucleic Acids Res,
36,
6633-6644.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Perera,
and
R.J.Kuhn
(2008).
Structural proteomics of dengue virus.
|
| |
Curr Opin Microbiol,
11,
369-377.
|
 |
|
|
|
|
 |
S.Chinnaswamy,
I.Yarbrough,
S.Palaninathan,
C.T.Kumar,
V.Vijayaraghavan,
B.Demeler,
S.M.Lemon,
J.C.Sacchettini,
and
C.C.Kao
(2008).
A locking mechanism regulates RNA synthesis and host protein interaction by the hepatitis C virus polymerase.
|
| |
J Biol Chem,
283,
20535-20546.
|
 |
|
|
|
|
 |
S.E.Galloway,
P.E.Richardson,
and
G.W.Wertz
(2008).
Analysis of a structural homology model of the 2'-O-ribose methyltransferase domain within the vesicular stomatitis virus L protein.
|
| |
Virology,
382,
69-82.
|
 |
|
|
|
|
 |
G.S.Park,
K.L.Morris,
R.G.Hallett,
M.E.Bloom,
and
S.M.Best
(2007).
Identification of residues critical for the interferon antagonist function of Langat virus NS5 reveals a role for the RNA-dependent RNA polymerase domain.
|
| |
J Virol,
81,
6936-6946.
|
 |
|
|
|
|
 |
M.De la Peña,
O.J.Kyrieleis,
and
S.Cusack
(2007).
Structural insights into the mechanism and evolution of the vaccinia virus mRNA cap N7 methyl-transferase.
|
| |
EMBO J,
26,
4913-4925.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.Beerens,
B.Selisko,
S.Ricagno,
I.Imbert,
L.van der Zanden,
E.J.Snijder,
and
B.Canard
(2007).
De novo initiation of RNA synthesis by the arterivirus RNA-dependent RNA polymerase.
|
| |
J Virol,
81,
8384-8395.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

