 |
PDBsum entry 2gwd

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of plant glutamate cysteine ligase in complex with mg2+ and l-glutamate
|
|
Structure:
|
 |
Glutamate cysteine ligase. Chain: a. Fragment: glutamate cysteine ligase. Synonym: gamma-glutamylcysteine synthetase, gamma-ecs, gcs. Engineered: yes
|
|
Source:
|
 |
Brassica juncea. Organism_taxid: 3707. Gene: gsh1. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
2.09Å
|
R-factor:
|
0.178
|
R-free:
|
0.227
|
|
|
Authors:
|
 |
M.Hothorn,A.Wachter,R.Gromes,T.Stuwe,T.Rausch,K.Scheffzek
|
Key ref:

|
 |
M.Hothorn
et al.
(2006).
Structural basis for the redox control of plant glutamate cysteine ligase.
J Biol Chem,
281,
27557-27565.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
04-May-06
|
Release date:
|
20-Jun-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O23736
(GSH1_BRAJU) -
Glutamate--cysteine ligase, chloroplastic from Brassica juncea
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
514 a.a.
436 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.2.2
- glutamate--cysteine ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-cysteine + L-glutamate + ATP = gamma-L-glutamyl-L-cysteine + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 L-cysteine
L-cysteine
|
+
|
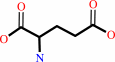 L-glutamate
L-glutamate
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
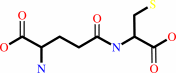 gamma-L-glutamyl-L-cysteine
gamma-L-glutamyl-L-cysteine
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
281:27557-27565
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for the redox control of plant glutamate cysteine ligase.
|
|
M.Hothorn,
A.Wachter,
R.Gromes,
T.Stuwe,
T.Rausch,
K.Scheffzek.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Glutathione (GSH) plays a crucial role in plant metabolism and stress response.
The rate-limiting step in the biosynthesis of GSH is catalyzed by glutamate
cysteine ligase (GCL) the activity of which is tightly regulated. The regulation
of plant GCLs is poorly understood. The crystal structure of substrate-bound GCL
from Brassica juncea at 2.1-A resolution reveals a plant-unique regulatory
mechanism based on two intramolecular redox-sensitive disulfide bonds. Reduction
of one disulfide bond allows a beta-hairpin motif to shield the active site of
B. juncea GCL, thereby preventing the access of substrates. Reduction of the
second disulfide bond reversibly controls dimer to monomer transition of B.
juncea GCL that is associated with a significant inactivation of the enzyme.
These regulatory events provide a molecular link between high GSH levels in the
plant cell and associated down-regulation of its biosynthesis. Furthermore,
known mutations in the Arabidopsis GCL gene affect residues in the close
proximity of the active site and thus explain the decreased GSH levels in mutant
plants. In particular, the mutation in rax1-1 plants causes impaired binding of
cysteine.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Plant GCL shows unique structural features. Front
and side views of BjGCL shown in ribbon representation. The
central  -sheet is depicted in
dark blue, the N- and C-terminal helical regions in light blue,
and the plant unique arms in dark and light green, respectively.
The L-glutamate bound in the active site is represented in bond
representation along with the Mg^2+ ion (in cyan). The two
disulfide bridges CC1 and CC2 are highlighted in yellow; the -sheet is depicted in
dark blue, the N- and C-terminal helical regions in light blue,
and the plant unique arms in dark and light green, respectively.
The L-glutamate bound in the active site is represented in bond
representation along with the Mg^2+ ion (in cyan). The two
disulfide bridges CC1 and CC2 are highlighted in yellow; the
 -hairpin module is shown
in red. -hairpin module is shown
in red.
|
 |
Figure 2.
FIGURE 2. Substrate binding in plant GCL. A, close-up view
of the glutamate binding site with the inhibitor BSO (in yellow;
sulfur depicted in magenta) in bond representation and including
the final 2F[obs] - F[calc] electron density map contoured at
1.5  . Residues reaching
from the central . Residues reaching
from the central  -sheet (in blue) to
coordinate the Mg^2+ ion (in cyan) are depicted in blue.
Residues contributed by the helical arms are shown in light
green. B, schematic representation of the inhibitor BSO binding
to BjGCL. The LigPlot diagram (50) summarizes key interactions
between the BSO ligand and active site residues. Yellow lines,
BSO ligand; green lines, BjGCL residues; semicircles with
radiating lines; atoms or residues involved in hydrophobic
contacts between protein and ligand. C, stereo close-up view of
the plant GCL cysteine binding pocket formed by mostly
hydrophobic residues (in blue) around the aliphatic side chain
of BSO (in light gray). The corresponding secondary structure
elements and residues in E. coli GCL (PDB-ID: 1VA6) are shown in
orange. D, known mutations in the Arabidopsis GCL gene are in
proximity of the substrate binding sites in plant GCL. BjGCL in
ribbon representation is shown with BSO and ADP (modeled) in
bonds representation (in yellow). Small spheres indicate the
positions of residues affected in AtGCL mutant plants (in
magenta). Enlarged versions provide models on how the affected
residues in rax1-1 and rml1 mutants may interact with GCL
substrates. The rax1-1 arginine residue (Arg^220) is shown in a
modeled rotamer configuration bringing its guanidinium group in
close proximity to the terminal methyl of BSO that corresponds
to the sulfhydryl group of cysteine (in green). Interactions are
highlighted by dotted lines (in magenta). -sheet (in blue) to
coordinate the Mg^2+ ion (in cyan) are depicted in blue.
Residues contributed by the helical arms are shown in light
green. B, schematic representation of the inhibitor BSO binding
to BjGCL. The LigPlot diagram (50) summarizes key interactions
between the BSO ligand and active site residues. Yellow lines,
BSO ligand; green lines, BjGCL residues; semicircles with
radiating lines; atoms or residues involved in hydrophobic
contacts between protein and ligand. C, stereo close-up view of
the plant GCL cysteine binding pocket formed by mostly
hydrophobic residues (in blue) around the aliphatic side chain
of BSO (in light gray). The corresponding secondary structure
elements and residues in E. coli GCL (PDB-ID: 1VA6) are shown in
orange. D, known mutations in the Arabidopsis GCL gene are in
proximity of the substrate binding sites in plant GCL. BjGCL in
ribbon representation is shown with BSO and ADP (modeled) in
bonds representation (in yellow). Small spheres indicate the
positions of residues affected in AtGCL mutant plants (in
magenta). Enlarged versions provide models on how the affected
residues in rax1-1 and rml1 mutants may interact with GCL
substrates. The rax1-1 arginine residue (Arg^220) is shown in a
modeled rotamer configuration bringing its guanidinium group in
close proximity to the terminal methyl of BSO that corresponds
to the sulfhydryl group of cysteine (in green). Interactions are
highlighted by dotted lines (in magenta).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2006,
281,
27557-27565)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
H.Takahashi,
S.Kopriva,
M.Giordano,
K.Saito,
and
R.Hell
(2011).
Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes.
|
| |
Annu Rev Plant Biol,
62,
157-184.
|
 |
|
|
|
|
 |
A.Koprivova,
S.T.Mugford,
and
S.Kopriva
(2010).
Arabidopsis root growth dependence on glutathione is linked to auxin transport.
|
| |
Plant Cell Rep,
29,
1157-1167.
|
 |
|
|
|
|
 |
H.Yi,
A.Galant,
G.E.Ravilious,
M.L.Preuss,
and
J.M.Jez
(2010).
Sensing sulfur conditions: simple to complex protein regulatory mechanisms in plant thiol metabolism.
|
| |
Mol Plant,
3,
269-279.
|
 |
|
|
|
|
 |
H.Yi,
G.E.Ravilious,
A.Galant,
H.B.Krishnan,
and
J.M.Jez
(2010).
From sulfur to homoglutathione: thiol metabolism in soybean.
|
| |
Amino Acids,
39,
963-978.
|
 |
|
|
|
|
 |
S.C.Maughan,
M.Pasternak,
N.Cairns,
G.Kiddle,
T.Brach,
R.Jarvis,
F.Haas,
J.Nieuwland,
B.Lim,
C.Müller,
E.Salcedo-Sora,
C.Kruse,
M.Orsel,
R.Hell,
A.J.Miller,
P.Bray,
C.H.Foyer,
J.A.Murray,
A.J.Meyer,
and
C.S.Cobbett
(2010).
Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses.
|
| |
Proc Natl Acad Sci U S A,
107,
2331-2336.
|
 |
|
|
|
|
 |
S.Krueger,
A.Donath,
M.C.Lopez-Martin,
R.Hoefgen,
C.Gotor,
and
H.Hesse
(2010).
Impact of sulfur starvation on cysteine biosynthesis in T-DNA mutants deficient for compartment-specific serine-acetyltransferase.
|
| |
Amino Acids,
39,
1029-1042.
|
 |
|
|
|
|
 |
V.Liedschulte,
A.Wachter,
A.Zhigang,
and
T.Rausch
(2010).
Exploiting plants for glutathione (GSH) production: Uncoupling GSH synthesis from cellular controls results in unprecedented GSH accumulation.
|
| |
Plant Biotechnol J,
8,
807-820.
|
 |
|
|
|
|
 |
C.C.Franklin,
D.S.Backos,
I.Mohar,
C.C.White,
H.J.Forman,
and
T.J.Kavanagh
(2009).
Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase.
|
| |
Mol Aspects Med,
30,
86-98.
|
 |
|
|
|
|
 |
C.H.Foyer,
G.Noctor,
B.Buchanan,
K.J.Dietz,
and
T.Pfannschmidt
(2009).
Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications.
|
| |
Antioxid Redox Signal,
11,
861-905.
|
 |
|
|
|
|
 |
E.I.Biterova,
and
J.J.Barycki
(2009).
Mechanistic details of glutathione biosynthesis revealed by crystal structures of Saccharomyces cerevisiae glutamate cysteine ligase.
|
| |
J Biol Chem,
284,
32700-32708.
|
 |
|
|
|
|
 |
J.Wu,
T.Qu,
S.Chen,
Z.Zhao,
and
L.An
(2009).
Molecular cloning and characterization of a gamma-glutamylcysteine synthetase gene from Chorispora bungeana.
|
| |
Protoplasma,
235,
27-36.
|
 |
|
|
|
|
 |
A.Figueiredo,
A.M.Fortes,
S.Ferreira,
M.Sebastiana,
Y.H.Choi,
L.Sousa,
B.Acioli-Santos,
F.Pessoa,
R.Verpoorte,
and
M.S.Pais
(2008).
Transcriptional and metabolic profiling of grape (Vitis vinifera L.) leaves unravel possible innate resistance against pathogenic fungi.
|
| |
J Exp Bot,
59,
3371-3381.
|
 |
|
|
|
|
 |
M.Pasternak,
B.Lim,
M.Wirtz,
R.Hell,
C.S.Cobbett,
and
A.J.Meyer
(2008).
Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development.
|
| |
Plant J,
53,
999.
|
 |
|
|
|
|
 |
N.Rouhier,
S.D.Lemaire,
and
J.P.Jacquot
(2008).
The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation.
|
| |
Annu Rev Plant Biol,
59,
143-166.
|
 |
|
|
|
|
 |
T.Rausch,
R.Gromes,
V.Liedschulte,
I.Müller,
J.Bogs,
V.Galovic,
and
A.Wachter
(2007).
Novel insight into the regulation of GSH biosynthesis in higher plants.
|
| |
Plant Biol (Stuttg),
9,
565-572.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

