 |
PDBsum entry 2c43

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Structure of aminoadipate-semialdehyde dehydrogenase- phosphopantetheinyl transferase in complex with coenzyme a
|
|
Structure:
|
 |
Aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase. Chain: a. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
1.93Å
|
R-factor:
|
0.162
|
R-free:
|
0.219
|
|
|
Authors:
|
 |
G.Bunkoczi,X.Wu,E.Dubinina,C.Johansson,C.Smee,A.Turnbull,F.Von Delft, C.Arrowsmith,A.Edwards,M.Sundstrom,J.Weigelt,U.Oppermann
|
|
Key ref:
|
 |
G.Bunkoczi
et al.
(2007).
Mechanism and substrate recognition of human holo ACP synthase.
Chem Biol,
14,
1243-1253.
PubMed id:
|
 |
|
Date:
|
 |
|
14-Oct-05
|
Release date:
|
24-Oct-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9NRN7
(ADPPT_HUMAN) -
L-aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
309 a.a.
280 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 6 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.8.7
- holo-[acyl-carrier-protein] synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
apo-[ACP] + CoA = holo-[ACP] + adenosine 3',5'-bisphosphate + H+
|
 |
 |
 |
 |
 |
apo-[ACP]
|
+
|
CoA
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
holo-[ACP]
|
+
|
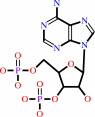 adenosine 3',5'-bisphosphate
adenosine 3',5'-bisphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Chem Biol
14:1243-1253
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Mechanism and substrate recognition of human holo ACP synthase.
|
|
G.Bunkoczi,
S.Pasta,
A.Joshi,
X.Wu,
K.L.Kavanagh,
S.Smith,
U.Oppermann.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Mammals utilize a single phosphopantetheinyl transferase for the
posttranslational modification of at least three different apoproteins: the
carrier protein components of cytosolic and mitochondrial fatty acid synthases
and the aminoadipate semialdehyde reductase involved in lysine degradation. We
determined the crystal structure of the human phosphopantetheinyl transferase, a
eukaryotic phosphopantetheinyl transferase characterized, complexed with CoA and
Mg(2+), and in ternary complex with CoA and ACP. The involvement of key residues
in ligand binding and catalysis was confirmed by mutagenesis and kinetic
analysis. Human phosphopantetheinyl transferase exhibits an alpha/beta fold and
2-fold pseudosymmetry similar to the Sfp phosphopantetheinyl transferase from
Bacillus subtilis. Although the bound ACP exhibits a typical four-helix
structure, its binding is unusual in that it is facilitated predominantly by
hydrophobic interactions. A detailed mechanism is proposed describing the
substrate binding and catalytic process.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.S.Halavaty,
Y.Kim,
G.Minasov,
L.Shuvalova,
I.Dubrovska,
J.Winsor,
M.Zhou,
O.Onopriyenko,
T.Skarina,
L.Papazisi,
K.Kwon,
S.N.Peterson,
A.Joachimiak,
A.Savchenko,
and
W.F.Anderson
(2012).
Structural characterization and comparison of three acyl-carrier-protein synthases from pathogenic bacteria.
|
| |
Acta Crystallogr D Biol Crystallogr,
68,
1359-1370.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.I.Chan,
and
H.J.Vogel
(2010).
Current understanding of fatty acid biosynthesis and the acyl carrier protein.
|
| |
Biochem J,
430,
1.
|
 |
|
|
|
|
 |
K.C.Strickland,
L.A.Hoeferlin,
N.V.Oleinik,
N.I.Krupenko,
and
S.A.Krupenko
(2010).
Acyl carrier protein-specific 4'-phosphopantetheinyl transferase activates 10-formyltetrahydrofolate dehydrogenase.
|
| |
J Biol Chem,
285,
1627-1633.
|
 |
|
|
|
|
 |
T.Maier,
M.Leibundgut,
D.Boehringer,
and
N.Ban
(2010).
Structure and function of eukaryotic fatty acid synthases.
|
| |
Q Rev Biophys,
43,
373-422.
|
 |
|
|
|
|
 |
E.J.Brignole,
S.Smith,
and
F.J.Asturias
(2009).
Conformational flexibility of metazoan fatty acid synthase enables catalysis.
|
| |
Nat Struct Mol Biol,
16,
190-197.
|
 |
|
|
|
|
 |
J.Cao,
H.Xu,
H.Zhao,
W.Gong,
and
D.Dunaway-Mariano
(2009).
The mechanisms of human hotdog-fold thioesterase 2 (hTHEM2) substrate recognition and catalysis illuminated by a structure and function based analysis.
|
| |
Biochemistry,
48,
1293-1304.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Leibundgut,
T.Maier,
S.Jenni,
and
N.Ban
(2008).
The multienzyme architecture of eukaryotic fatty acid synthases.
|
| |
Curr Opin Struct Biol,
18,
714-725.
|
 |
|
|
|
|
 |
S.E.Evans,
C.Williams,
C.J.Arthur,
S.G.Burston,
T.J.Simpson,
J.Crosby,
and
M.P.Crump
(2008).
An ACP structural switch: conformational differences between the apo and holo forms of the actinorhodin polyketide synthase acyl carrier protein.
|
| |
Chembiochem,
9,
2424-2432.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Maier,
M.Leibundgut,
and
N.Ban
(2008).
The crystal structure of a mammalian fatty acid synthase.
|
| |
Science,
321,
1315-1322.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

