 |
PDBsum entry 1x9h

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.5.3.1.8
- mannose-6-phosphate isomerase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
GDP-L-Fucose and GDP-mannose Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-mannose 6-phosphate = D-fructose 6-phosphate
|
 |
 |
 |
 |
 |
D-mannose 6-phosphate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
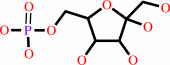 D-fructose 6-phosphate
D-fructose 6-phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.5.3.1.9
- glucose-6-phosphate isomerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
alpha-D-glucose 6-phosphate = beta-D-fructose 6-phosphate
|
 |
 |
 |
 |
 |
alpha-D-glucose 6-phosphate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
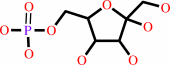 beta-D-fructose 6-phosphate
beta-D-fructose 6-phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
43:14088-14095
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for phosphomannose isomerase activity in phosphoglucose isomerase from Pyrobaculum aerophilum: a subtle difference between distantly related enzymes.
|
|
M.K.Swan,
T.Hansen,
P.Schönheit,
C.Davies.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of a dual-specificity phosphoglucose/phosphomannose
isomerase from the crenarchaeon Pyrobaculum aerophilum (PaPGI/PMI) has been
determined in complex with glucose 6-phosphate at 1.16 A resolution and with
fructose 6-phosphate at 1.5 A resolution. Subsequent modeling of mannose
6-phosphate (M6P) into the active site of the enzyme shows that the PMI activity
of this enzyme may be due to the additional space imparted by a threonine. In
PGIs from bacterial and eukaryotic sources, which cannot use M6P as a substrate,
the equivalent residue is a glutamine. The increased space may permit rotation
of the C2-C3 bond in M6P to facilitate abstraction of a proton from C2 by Glu203
and, after a further C2-C3 rotation of the resulting cis-enediolate, re-donation
of a proton to C1 by the same residue. A proline residue (in place of a glycine
in PGI) may also promote PMI activity by positioning the C1-O1 region of M6P.
Thus, the PMI reaction in PaPGI/PMI probably uses a cis-enediol mechanism of
catalysis, and this activity appears to arise from a subtle difference in the
architecture of the enzyme, compared to bacterial and eukaryotic PGIs.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Roux,
F.Bhatt,
J.Foret,
B.de Courcy,
N.Gresh,
J.P.Piquemal,
C.J.Jeffery,
and
L.Salmon
(2011).
The reaction mechanism of type I phosphomannose isomerases: new information from inhibition and polarizable molecular mechanics studies.
|
| |
Proteins,
79,
203-220.
|
 |
|
|
|
|
 |
S.J.Yeom,
J.H.Ji,
N.H.Kim,
C.S.Park,
and
D.K.Oh
(2009).
Substrate specificity of a mannose-6-phosphate isomerase from Bacillus subtilis and its application in the production of L-ribose.
|
| |
Appl Environ Microbiol,
75,
4705-4710.
|
 |
|
|
|
|
 |
S.R.Sagurthi,
G.Gowda,
H.S.Savithri,
and
M.R.Murthy
(2009).
Structures of mannose-6-phosphate isomerase from Salmonella typhimurium bound to metal atoms and substrate: implications for catalytic mechanism.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
724-732.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Roux,
N.Gresh,
L.E.Perera,
J.P.Piquemal,
and
L.Salmon
(2007).
Binding of 5-phospho-D-arabinonohydroxamate and 5-phospho-D-arabinonate inhibitors to zinc phosphomannose isomerase from Candida albicans studied by polarizable molecular mechanics and quantum mechanics.
|
| |
J Comput Chem,
28,
938-957.
|
 |
|
|
|
|
 |
M.Reher,
S.Gebhard,
and
P.Schönheit
(2007).
Glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) and nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN), key enzymes of the respective modified Embden-Meyerhof pathways in the hyperthermophilic crenarchaeota Pyrobaculum aerophilum and Aeropyrum pernix.
|
| |
FEMS Microbiol Lett,
273,
196-205.
|
 |
|
|
|
|
 |
B.Siebers,
and
P.Schönheit
(2005).
Unusual pathways and enzymes of central carbohydrate metabolism in Archaea.
|
| |
Curr Opin Microbiol,
8,
695-705.
|
 |
|
|
|
|
 |
T.Hansen,
B.Schlichting,
J.Grötzinger,
M.K.Swan,
C.Davies,
and
P.Schönheit
(2005).
Mutagenesis of catalytically important residues of cupin type phosphoglucose isomerase from Archaeoglobus fulgidus.
|
| |
FEBS J,
272,
6266-6275.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

