
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|





 (+ 4 more)
218 a.a.
(+ 4 more)
218 a.a.
|
 |

|

|

|

|

|

|

|





 (+ 4 more)
310 a.a.
(+ 4 more)
310 a.a.
|
 |

|

|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Paf-ah holoenzyme: lis1/alfa2
|
|
Structure:
|
 |
Platelet-activating factor acetylhydrolase ib beta subunit. Chain: a, b, e, f, i, j, m, n, q, r. Synonym: platelet-activating factor acetylhydrolase, paf acetylhydrolase 30 kda subunit, paf-ah 30 kda subunit, paf-ah beta subunit, pafah beta subunit. Engineered: yes. Platelet-activating factor acetylhydrolase ib alpha subunit. Chain: c, d, g, h, k, l, o, p, s, t.
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Expressed in: escherichia coli. Expression_system_taxid: 469008. Mus musculus. Mouse. Organism_taxid: 10090. Organ: brain.
|
|
Biol. unit:
|
 |
 Tetramer (from PDB file)
Tetramer (from PDB file)
|
|
Resolution:
|
 |
|
3.40Å
|
R-factor:
|
0.265
|
R-free:
|
0.307
|
|
|
Authors:
|
 |
C.Tarricone,F.Perrina,S.Monzani,L.Massimiliano,S.Knapp,L.-H.Tsai, Z.S.Derewenda,A.Musacchio
|
Key ref:
|
 |
C.Tarricone
et al.
(2004).
Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase.
Neuron,
44,
809-821.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
30-Apr-04
|
Release date:
|
26-May-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
Chains A, B, E, F, I, J, M, N, Q, R:
E.C.3.1.1.47
- 1-alkyl-2-acetylglycerophosphocholine esterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine + H2O = a 1-O-alkyl- sn-glycero-3-phosphocholine + acetate + H+
|
 |
 |
 |
 |
 |
1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
1-O-alkyl- sn-glycero-3-phosphocholine
|
+
|
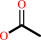 acetate
acetate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains C, D, G, H, K, L, O, P, S, T:
E.C.?
|
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Neuron
44:809-821
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase.
|
|
C.Tarricone,
F.Perrina,
S.Monzani,
L.Massimiliano,
M.H.Kim,
Z.S.Derewenda,
S.Knapp,
L.H.Tsai,
A.Musacchio.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Mutations in the LIS1 gene cause lissencephaly, a human neuronal migration
disorder. LIS1 binds dynein and the dynein-associated proteins Nde1 (formerly
known as NudE), Ndel1 (formerly known as NUDEL), and CLIP-170, as well as the
catalytic alpha dimers of brain cytosolic platelet activating factor
acetylhydrolase (PAF-AH). The mechanism coupling the two diverse regulatory
pathways remains unknown. We report the structure of LIS1 in complex with the
alpha2/alpha2 PAF-AH homodimer. One LIS1 homodimer binds symmetrically to one
alpha2/alpha2 homodimer via the highly conserved top faces of the LIS1 beta
propellers. The same surface of LIS1 contains sites of mutations causing
lissencephaly and overlaps with a putative dynein binding surface. Ndel1
competes with the alpha2/alpha2 homodimer for LIS1, but the interaction is
complex and requires both the N- and C-terminal domains of LIS1. Our data
suggest that the LIS1 molecule undergoes major conformational rearrangement when
switching from a complex with the acetylhydrolase to the one with Ndel1.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Comparison of LIS1/PAF-AH and G[αβγ](A) Ribbon
diagram of the G[αβγ] trimer based on PDB coordinates 1GP2
(Wall et al., 1995). G[α] is yellow and is subdivided in the
Ras-like domain (light yellow) and in the helical domain (dark
yellow). G[β] is cyan and G[γ] is green. The G[α]-G[βγ]
interaction involves the N-terminal helix and the switch I and
switch II regions of G[α] (red). The G[β] subunit uses the top
surface of the β propeller, where the outer βD strand connects
to the inner βA strand of the next blade to bind G[α]. The
top surface is preferentially used for ligand recognition by
propeller-like structures (Smith et al., 1999).(B) A LIS1/PAF-AH
hemitetramer is shown. The α[2] subunit has the same
orientation of the G[α] subunit shown in (A). The orientation
of the β subunits is unrelated, although also in this case the
top surface of the propeller is used for binding.
|
 |
Figure 7.
Figure 7. A Model for LIS1/Ndel1 and Its Interacton with
DyneinTo reconcile the two-fold symmetry of LIS1 with that of
Ndel1, we postulated that Ndel1 forms an antiparallel coiled
coil. The α4 helices of N-LIS1 interact with Ndel1, and so does
the β propeller region. The two β propellers of LIS1 bind
dynein at distinct sites in the first AAA module and in the stem
(Tai et al., 2002). Because the dynein heavy chain that
contributes a sizable fraction of the stem is a dimer (Holzbaur
and Vallee, 1994), the overall assembly may be duplicated
(represented by the gray, dashed drawing).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Neuron
(2004,
44,
809-821)
copyright 2004.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Zyłkiewicz,
M.Kijańska,
W.C.Choi,
U.Derewenda,
Z.S.Derewenda,
and
P.T.Stukenberg
(2011).
The N-terminal coiled-coil of Ndel1 is a regulated scaffold that recruits LIS1 to dynein.
|
| |
J Cell Biol,
192,
433-445.
|
 |
|
|
|
|
 |
S.M.Markus,
K.M.Plevock,
B.J.St Germain,
J.J.Punch,
C.W.Meaden,
and
W.L.Lee
(2011).
Quantitative analysis of Pac1/LIS1-mediated dynein targeting: Implications for regulation of dynein activity in budding yeast.
|
| |
Cytoskeleton (Hoboken),
68,
157-174.
|
 |
|
|
|
|
 |
M.E.Bechler,
A.M.Doody,
E.Racoosin,
L.Lin,
K.H.Lee,
and
W.J.Brown
(2010).
The phospholipase complex PAFAH Ib regulates the functional organization of the Golgi complex.
|
| |
J Cell Biol,
190,
45-53.
|
 |
|
|
|
|
 |
R.J.McKenney,
M.Vershinin,
A.Kunwar,
R.B.Vallee,
and
S.P.Gross
(2010).
LIS1 and NudE induce a persistent dynein force-producing state.
|
| |
Cell,
141,
304-314.
|
 |
|
|
|
|
 |
Y.Yang,
X.Yan,
Y.Cai,
Y.Lu,
J.Si,
and
T.Zhou
(2010).
NudC-like protein 2 regulates the LIS1/dynein pathway by stabilizing LIS1 with Hsp90.
|
| |
Proc Natl Acad Sci U S A,
107,
3499-3504.
|
 |
|
|
|
|
 |
G.Zhang,
A.H.Assadi,
M.Roceri,
G.D.Clark,
and
G.D'Arcangelo
(2009).
Differential interaction of the Pafah1b alpha subunits with the Reelin transducer Dab1.
|
| |
Brain Res,
1267,
1-8.
|
 |
|
|
|
|
 |
J.R.Kardon,
and
R.D.Vale
(2009).
Regulators of the cytoplasmic dynein motor.
|
| |
Nat Rev Mol Cell Biol,
10,
854-865.
|
 |
|
|
|
|
 |
R.B.Vallee,
G.E.Seale,
and
J.W.Tsai
(2009).
Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones.
|
| |
Trends Cell Biol,
19,
347-355.
|
 |
|
|
|
|
 |
T.M.Epstein,
U.Samanta,
S.D.Kirby,
D.M.Cerasoli,
and
B.J.Bahnson
(2009).
Crystal structures of brain group-VIII phospholipase A2 in nonaged complexes with the organophosphorus nerve agents soman and sarin.
|
| |
Biochemistry,
48,
3425-3435.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Akhmanova,
and
M.O.Steinmetz
(2008).
Tracking the ends: a dynamic protein network controls the fate of microtubule tips.
|
| |
Nat Rev Mol Cell Biol,
9,
309-322.
|
 |
|
|
|
|
 |
H.Ullah,
E.L.Scappini,
A.F.Moon,
L.V.Williams,
D.L.Armstrong,
and
L.C.Pedersen
(2008).
Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana.
|
| |
Protein Sci,
17,
1771-1780.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Helmstaedt,
K.Laubinger,
K.Vosskuhl,
O.Bayram,
S.Busch,
M.Hoppert,
O.Valerius,
S.Seiler,
and
G.H.Braus
(2008).
The nuclear migration protein NUDF/LIS1 forms a complex with NUDC and BNFA at spindle pole bodies.
|
| |
Eukaryot Cell,
7,
1041-1052.
|
 |
|
|
|
|
 |
S.Hebbar,
M.T.Mesngon,
A.M.Guillotte,
B.Desai,
R.Ayala,
and
D.S.Smith
(2008).
Lis1 and Ndel1 influence the timing of nuclear envelope breakdown in neural stem cells.
|
| |
J Cell Biol,
182,
1063-1071.
|
 |
|
|
|
|
 |
S.Y.Shim,
B.A.Samuels,
J.Wang,
G.Neumayer,
C.Belzil,
R.Ayala,
Y.Shi,
Y.Shi,
L.H.Tsai,
and
M.D.Nguyen
(2008).
Ndel1 controls the dynein-mediated transport of vimentin during neurite outgrowth.
|
| |
J Biol Chem,
283,
12232-12240.
|
 |
|
|
|
|
 |
U.Samanta,
and
B.J.Bahnson
(2008).
Crystal Structure of Human Plasma Platelet-activating Factor Acetylhydrolase: STRUCTURAL IMPLICATION TO LIPOPROTEIN BINDING AND CATALYSIS.
|
| |
J Biol Chem,
283,
31617-31624.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Yamaguchi,
H.Koizumi,
J.Aoki,
Y.Natori,
K.Nishikawa,
Y.Natori,
Y.Takanezawa,
and
H.Arai
(2007).
Type I platelet-activating factor acetylhydrolase catalytic subunits over-expression induces pleiomorphic nuclei and centrosome amplification.
|
| |
Genes Cells,
12,
1153-1161.
|
 |
|
|
|
|
 |
A.Pramatarova,
P.G.Ochalski,
C.H.Lee,
and
B.W.Howell
(2006).
Mouse disabled 1 regulates the nuclear position of neurons in a Drosophila eye model.
|
| |
Mol Cell Biol,
26,
1510-1517.
|
 |
|
|
|
|
 |
A.Schuetz,
A.Allali-Hassani,
F.Martín,
P.Loppnau,
M.Vedadi,
A.Bochkarev,
A.N.Plotnikov,
C.H.Arrowsmith,
and
J.Min
(2006).
Structural basis for molecular recognition and presentation of histone H3 by WDR5.
|
| |
EMBO J,
25,
4245-4252.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Gressens
(2006).
Pathogenesis of migration disorders.
|
| |
Curr Opin Neurol,
19,
135-140.
|
 |
|
|
|
|
 |
A.Kamiya,
K.Kubo,
T.Tomoda,
M.Takaki,
R.Youn,
Y.Ozeki,
N.Sawamura,
U.Park,
C.Kudo,
M.Okawa,
C.A.Ross,
M.E.Hatten,
K.Nakajima,
and
A.Sawa
(2005).
A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development.
|
| |
Nat Cell Biol,
7,
1167-1178.
|
 |
|
|
|
|
 |
J.Li,
W.L.Lee,
and
J.A.Cooper
(2005).
NudEL targets dynein to microtubule ends through LIS1.
|
| |
Nat Cell Biol,
7,
686-690.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

