 |
PDBsum entry 1v3t

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1v3t
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Crystal structure of leukotriene b4 12-hydroxydehydrogenase/15-oxo- prostaglandin 13-reductase
|
|
Structure:
|
 |
Leukotriene b4 12-hydroxydehydrogenase/prostaglandin 15- keto reductase. Chain: a, b. Synonym: leukotriene b4 12-hydroxydehydrogenase/15-oxo-prostaglandin 13-reductase. Engineered: yes
|
|
Source:
|
 |
Cavia porcellus. Domestic guinea pig. Organism_taxid: 10141. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
2.30Å
|
R-factor:
|
0.194
|
R-free:
|
0.255
|
|
|
Authors:
|
 |
T.Hori,T.Yokomizo,H.Ago,M.Sugahara,G.Ueno,M.Yamamoto,T.Kumasaka, T.Shimizu,M.Miyano,Riken Structural Genomics/proteomics Initiative (Rsgi)
|
Key ref:

|
 |
T.Hori
et al.
(2004).
Structural basis of leukotriene B4 12-hydroxydehydrogenase/15-Oxo-prostaglandin 13-reductase catalytic mechanism and a possible Src homology 3 domain binding loop.
J Biol Chem,
279,
22615-22623.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
05-Nov-03
|
Release date:
|
13-Jul-04
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9EQZ5
(PTGR1_CAVPO) -
Prostaglandin reductase 1 from Cavia porcellus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
329 a.a.
333 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.1.3.1.48
- 15-oxoprostaglandin 13-reductase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
13,14-dihydro-15-oxo-prostaglandin E2 + NADP+ = 15-oxoprostaglandin E2 + NADPH + H+
|
|
2.
|
13,14-dihydro-15-oxo-prostaglandin E2 + NAD+ = 15-oxoprostaglandin E2 + NADH + H+
|
|
 |
 |
 |
 |
 |
13,14-dihydro-15-oxo-prostaglandin E2
|
+
|
NADP(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
15-oxoprostaglandin E2
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
13,14-dihydro-15-oxo-prostaglandin E2
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
=
|
15-oxoprostaglandin E2
|
+
|
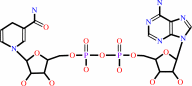 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.3.1.74
- 2-alkenal reductase [NAD(P)(+)].
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
an n-alkanal + NAD+ = an alk-2-enal + NADH + H+
|
|
2.
|
an n-alkanal + NADP+ = an alk-2-enal + NADPH + H+
|
|
 |
 |
 |
 |
 |
n-alkanal
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
=
|
alk-2-enal
|
+
|
 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
n-alkanal
|
+
|
NADP(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
alk-2-enal
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
279:22615-22623
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis of leukotriene B4 12-hydroxydehydrogenase/15-Oxo-prostaglandin 13-reductase catalytic mechanism and a possible Src homology 3 domain binding loop.
|
|
T.Hori,
T.Yokomizo,
H.Ago,
M.Sugahara,
G.Ueno,
M.Yamamoto,
T.Kumasaka,
T.Shimizu,
M.Miyano.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The bifunctional leukotriene B(4) 12-hydroxydehydrogenase/15-oxo-prostaglandin
13-reductase (LTB(4) 12-HD/PGR) is an essential enzyme for eicosanoid
inactivation. It is involved in the metabolism of the E and F series of
15-oxo-prostaglandins (15-oxo-PGs), leukotriene B(4) (LTB(4)), and
15-oxo-lipoxin A(4) (15-oxo-LXA(4)). Some nonsteroidal anti-inflammatory drugs
(NSAIDs), which primarily act as cyclooxygenase inhibitors also inhibit LTB(4)
12-HD/PGR activity. Here we report the crystal structure of the LTB(4)
12-HD/PGR, the binary complex structure with NADP(+), and the ternary complex
structure with NADP(+) and 15-oxo-PGE(2). In the ternary complex, both in the
crystalline form and in solution, the enolate anion intermediate accumulates as
a brown chromophore. PGE(2) contains two chains, but only the omega-chain of
15-oxo-PGE(2) was defined in the electron density map in the ternary complex
structure. The omega-chain was identified at the hydrophobic pore on the dimer
interface. The structure showed that the 15-oxo group forms hydrogen bonds with
the 2'-hydroxyl group of nicotine amide ribose of NADP(+) and a bound water
molecule to stabilize the enolate intermediate during the reductase reaction.
The electron-deficient C13 atom of the conjugated enolate may be directly
attacked by a hydride from the NADPH nicotine amide in a stereospecific manner.
The moderate recognition of 15-oxo-PGE(2) is consistent with a broad substrate
specificity of LTB(4) 12-HD/PGR. The structure also implies that a Src homology
domain 3 may interact with the left-handed proline-rich helix at the dimer
interface and regulate LTB(4) 12-HD/PGR activity by disruption of the substrate
binding pore to accommodate the omega-chain.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
FIG. 3. NADP+ and 15-oxo-PGE[2] binding to LTB[4]
12-HD/PGR. A, a stereo view of the bound NADP+ in the binary
complex structure. The|F[o]| -|F[c]| simulated annealing omit
electron density map is contoured at 3.0  (orange) and 6.0 (orange) and 6.0  (cyan).
The carbon atoms of NADP+ are yellow, and those of side chains
involved in NADP+ binding are green. Single-letter codes are
used for amino acid residues. The water molecules, oxygen,
nitrogen, and phosphorus atoms are colored pink, red, blue, and
orange, respectively. The hydrogen bonds are indicated by dashed
lines. The main chain nitrogen or carbonyl groups interacting
with the bound NADP+ are labeled by residue numbers. B, a photo
of the ternary complex crystals (0.3 x 0.2 x 0.1 mm). C,
absorption spectrum changes of a ternary complex solution
mixture. A 1:10 dilution of the ternary mixture after NaOH
addition was measured. D, stereo view of the (cyan).
The carbon atoms of NADP+ are yellow, and those of side chains
involved in NADP+ binding are green. Single-letter codes are
used for amino acid residues. The water molecules, oxygen,
nitrogen, and phosphorus atoms are colored pink, red, blue, and
orange, respectively. The hydrogen bonds are indicated by dashed
lines. The main chain nitrogen or carbonyl groups interacting
with the bound NADP+ are labeled by residue numbers. B, a photo
of the ternary complex crystals (0.3 x 0.2 x 0.1 mm). C,
absorption spectrum changes of a ternary complex solution
mixture. A 1:10 dilution of the ternary mixture after NaOH
addition was measured. D, stereo view of the  -chain of bound
15-oxo-PGE[2] in the ternary complex structure. The carbon atoms
of the -chain of bound
15-oxo-PGE[2] in the ternary complex structure. The carbon atoms
of the  -chain of 15-oxo-PGE[2]
are colored in cyan, and NADP+ in yellow. The residues from the
same subunit of the bound NADP+ around the -chain of 15-oxo-PGE[2]
are colored in cyan, and NADP+ in yellow. The residues from the
same subunit of the bound NADP+ around the  -chain hydrophobic pore
are colored in green, and those from the other subunit are in
magenta.|F[o]| -|F[c]| simulated annealing omit electron density
map is contoured at 2.4 -chain hydrophobic pore
are colored in green, and those from the other subunit are in
magenta.|F[o]| -|F[c]| simulated annealing omit electron density
map is contoured at 2.4  (orange) and 3.5 (orange) and 3.5  (blue).
The residues Ala-241, Tyr-245, and Met-248 are located on the
loop (blue).
The residues Ala-241, Tyr-245, and Met-248 are located on the
loop  E- E-  F. Tyr-273 was refined
as two conformers. E, a stereo view of the interaction between
the F. Tyr-273 was refined
as two conformers. E, a stereo view of the interaction between
the  -chain and NADP+. The
hydrogen bonds and the -chain and NADP+. The
hydrogen bonds and the  - -  orbital interactions
between the nicotine amide ring and the conjugated double bonds
of the orbital interactions
between the nicotine amide ring and the conjugated double bonds
of the  -chain are indicated in
dashed lines. The shortest distance of the -chain are indicated in
dashed lines. The shortest distance of the  - -  orbital interactions is
3.2 Å between the carbonyl oxygen atom of 15-oxo-PGE[2]
and the nitrogen atom of the nicotine amide ring. orbital interactions is
3.2 Å between the carbonyl oxygen atom of 15-oxo-PGE[2]
and the nitrogen atom of the nicotine amide ring.
|
 |
Figure 5.
FIG. 5. Schematic drawing of the enolate anion intermediate
in 15-oxo-PGE[2] reductase reaction mechanism. Only the  -chain
and the cyclopentane ring are represented (see "Results and
Discussion" for further details). -chain
and the cyclopentane ring are represented (see "Results and
Discussion" for further details).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2004,
279,
22615-22623)
copyright 2004.
|
|
| |
Figures were
selected
by the author.
|
|

|
| |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|

|
| |
The LTB[4] 12-HD/PGR inhibitor, indomethacin complex was reported. (Hori, T. et al., J Biochem (Tokyo). (2006, 140,457-66)).
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Hassaninasab,
Y.Hashimoto,
K.Tomita-Yokotani,
and
M.Kobayashi
(2011).
Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism.
|
| |
Proc Natl Acad Sci U S A,
108,
6615-6620.
|
 |
|
|
|
|
 |
C.Shionyu-Mitsuyama,
T.Waku,
T.Shiraki,
T.Oyama,
T.Shirai,
and
K.Morikawa
(2011).
Detecting structural similarity of ligand interactions in the lipid metabolic system including enzymes, lipid-binding proteins and nuclear receptors.
|
| |
Protein Eng Des Sel,
24,
397-403.
|
 |
|
|
|
|
 |
H.Fujino,
and
T.Murayama
(2011).
[Novel anti-cancer effects of indomethacin: exploring the cyclooxygenase-inhibition-independent effects].
|
| |
Nippon Yakurigaku Zasshi,
137,
177-181.
|
 |
|
|
|
|
 |
S.Porté,
A.Moeini,
I.Reche,
N.Shafqat,
U.Oppermann,
J.Farrés,
and
X.Parés
(2011).
Kinetic and structural evidence of the alkenal/one reductase specificity of human ζ-crystallin.
|
| |
Cell Mol Life Sci,
68,
1065-1077.
|
 |
|
|
|
|
 |
K.Okita,
S.Motohashi,
R.Shinnakasu,
K.Nagato,
K.Yamasaki,
Y.Sato,
H.Kitamura,
A.Hijikata,
M.Yamashita,
K.Shimizu,
S.Fujii,
O.Ohara,
M.Taniguchi,
I.Sakaida,
and
T.Nakayama
(2010).
A set of genes associated with the interferon-γ response of lung cancer patients undergoing α-galactosylceramide-pulsed dendritic cell therapy.
|
| |
Cancer Sci,
101,
2333-2340.
|
 |
|
|
|
|
 |
M.W.Buczynski,
D.S.Dumlao,
and
E.A.Dennis
(2009).
Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology.
|
| |
J Lipid Res,
50,
1015-1038.
|
 |
|
|
|
|
 |
S.Porté,
E.Valencia,
E.A.Yakovtseva,
E.Borràs,
N.Shafqat,
J.E.Debreczeny,
A.C.Pike,
U.Oppermann,
J.Farrés,
I.Fita,
and
X.Parés
(2009).
Three-dimensional structure and enzymatic function of proapoptotic human p53-inducible quinone oxidoreductase PIG3.
|
| |
J Biol Chem,
284,
17194-17205.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Tsuchiya,
Y.Tachida,
E.Segi-Nishida,
Y.Okuno,
S.Tamba,
G.Tsujimoto,
S.Tanaka,
and
Y.Sugimoto
(2009).
Characterization of gene expression profiles for different types of mast cells pooled from mouse stomach subregions by an RNA amplification method.
|
| |
BMC Genomics,
10,
35.
|
 |
|
|
|
|
 |
B.Persson,
J.Hedlund,
and
H.Jörnvall
(2008).
Medium- and short-chain dehydrogenase/reductase gene and protein families : the MDR superfamily.
|
| |
Cell Mol Life Sci,
65,
3879-3894.
|
 |
|
|
|
|
 |
L.H.Heckmann,
R.M.Sibly,
M.J.Timmermans,
and
A.Callaghan
(2008).
Outlining eicosanoid biosynthesis in the crustacean Daphnia.
|
| |
Front Zool,
5,
11.
|
 |
|
|
|
|
 |
L.H.Heckmann,
R.M.Sibly,
R.Connon,
H.L.Hooper,
T.H.Hutchinson,
S.J.Maund,
C.J.Hill,
A.Bouetard,
and
A.Callaghan
(2008).
Systems biology meets stress ecology: linking molecular and organismal stress responses in Daphnia magna.
|
| |
Genome Biol,
9,
R40.
|
 |
|
|
|
|
 |
R.C.De Lisle,
L.Meldi,
M.Flynn,
and
K.Jansson
(2008).
Altered eicosanoid metabolism in the cystic fibrosis mouse small intestine.
|
| |
J Pediatr Gastroenterol Nutr,
47,
406-416.
|
 |
|
|
|
|
 |
T.Maier,
M.Leibundgut,
and
N.Ban
(2008).
The crystal structure of a mammalian fatty acid synthase.
|
| |
Science,
321,
1315-1322.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.H.Wu,
T.P.Ko,
R.T.Guo,
S.M.Hu,
L.M.Chuang,
and
A.H.Wang
(2008).
Structural basis for catalytic and inhibitory mechanisms of human prostaglandin reductase PTGR2.
|
| |
Structure,
16,
1714-1723.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
U.Oppermann
(2007).
Carbonyl reductases: the complex relationships of mammalian carbonyl- and quinone-reducing enzymes and their role in physiology.
|
| |
Annu Rev Pharmacol Toxicol,
47,
293-322.
|
 |
|
|
|
|
 |
G.Ueno,
H.Kanda,
R.Hirose,
K.Ida,
T.Kumasaka,
and
M.Yamamoto
(2006).
RIKEN structural genomics beamlines at the SPring-8; high throughput protein crystallography with automated beamline operation.
|
| |
J Struct Funct Genomics,
7,
15-22.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

