 |
PDBsum entry 1a6x

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Carrier protein
|
PDB id
|
|

|

|
1a6x
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.4.1.2
- acetyl-CoA carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydrogencarbonate + acetyl-CoA + ATP = malonyl-CoA + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 hydrogencarbonate
hydrogencarbonate
|
+
|
 acetyl-CoA
acetyl-CoA
|
+
|
 ATP
ATP
|
=
|
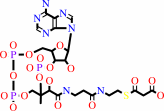 malonyl-CoA
malonyl-CoA
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Biotin
|
 |
 |
 |
 |
 |
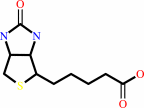 Biotin
Biotin
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
36:15089-15100
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the carboxy-terminal fragment of the apo-biotin carboxyl carrier subunit of Escherichia coli acetyl-CoA carboxylase.
|
|
X.Yao,
D.Wei,
C.Soden,
M.F.Summers,
D.Beckett.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The biotin carboxyl carrier protein (BCCP) is a subunit of acetyl-CoA
carboxylase, a biotin-dependent enzyme that catalyzes the first committed step
of fatty acid biosynthesis. In its functional cycle the biotin carboxyl carrier
protein engages in heterologous protein-protein interactions with three distinct
partners, depending on its state of posttranslational modification. Apo-BCCP
interacts specifically with the biotin holoenzyme synthetase, BirA, which
results in the posttranslational attachment of biotin to an essential lysine
residue on BCCP. Holo-BCCP then interacts with the biotin carboxylase subunit,
which leads to the addition of the carboxylate group of bicarbonate to biotin.
Finally, the carboxybiotinylated form of BCCP interacts with transcarboxylase in
the conversion of acetyl-CoA to malonyl-CoA. The determinants of protein-protein
interaction specificity in this system are unknown. One hypothesis is that
posttranslational modification of BCCP may result in conformational changes that
regulate specific protein-protein interactions. To test this hypothesis, we have
determined the NMR solution structure of the unbiotinylated form of an 87
residue C-terminal domain fragment of BCCP (apoBCCP87) from Escherichia coli
acetyl-CoA carboxylase and compared this structure with the high-resolution
structure of the biotinylated form that was recently solved by X-ray
crystallographic techniques. Although the overall folding of the two proteins is
highly similar, small structural differences are apparent for residues of the
biotin-binding loop that may be important for mediating specific protein-protein
interactions.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
G.Gago,
L.Diacovich,
A.Arabolaza,
S.C.Tsai,
and
H.Gramajo
(2011).
Fatty acid biosynthesis in actinomycetes.
|
| |
FEMS Microbiol Rev,
35,
475-497.
|
 |
|
|
|
|
 |
C.K.Lee,
H.K.Cheong,
K.S.Ryu,
J.I.Lee,
W.Lee,
Y.H.Jeon,
and
C.Cheong
(2008).
Biotinoyl domain of human acetyl-CoA carboxylase: Structural insights into the carboxyl transfer mechanism.
|
| |
Proteins,
72,
613-624.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.Lazzeri,
P.Erba,
M.Perri,
C.Tascini,
R.Doria,
J.Giorgetti,
and
G.Mariani
(2008).
Scintigraphic imaging of vertebral osteomyelitis with 111in-biotin.
|
| |
Spine,
33,
E198-E204.
|
 |
|
|
|
|
 |
D.Beckett
(2007).
Biotin sensing: universal influence of biotin status on transcription.
|
| |
Annu Rev Genet,
41,
443-464.
|
 |
|
|
|
|
 |
E.D.Streaker,
and
D.Beckett
(2006).
Nonenzymatic biotinylation of a biotin carboxyl carrier protein: unusual reactivity of the physiological target lysine.
|
| |
Protein Sci,
15,
1928-1935.
|
 |
|
|
|
|
 |
G.Cui,
B.Nan,
J.Hu,
Y.Wang,
C.Jin,
and
B.Xia
(2006).
Identification and solution structures of a single domain biotin/lipoyl attachment protein from Bacillus subtilis.
|
| |
J Biol Chem,
281,
20598-20607.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.Beckett
(2005).
Multilevel regulation of protein-protein interactions in biological circuitry.
|
| |
Phys Biol,
2,
S67-S73.
|
 |
|
|
|
|
 |
H.S.Kim,
U.Hoja,
J.Stolz,
G.Sauer,
and
E.Schweizer
(2004).
Identification of the tRNA-binding protein Arc1p as a novel target of in vivo biotinylation in Saccharomyces cerevisiae.
|
| |
J Biol Chem,
279,
42445-42452.
|
 |
|
|
|
|
 |
D.J.Clarke,
J.Coulson,
R.Baillie,
and
D.J.Campopiano
(2003).
Biotinylation in the hyperthermophile Aquifex aeolicus.
|
| |
Eur J Biochem,
270,
1277-1287.
|
 |
|
|
|
|
 |
E.Choi-Rhee,
and
J.E.Cronan
(2003).
The biotin carboxylase-biotin carboxyl carrier protein complex of Escherichia coli acetyl-CoA carboxylase.
|
| |
J Biol Chem,
278,
30806-30812.
|
 |
|
|
|
|
 |
S.Chuakrut,
H.Arai,
M.Ishii,
and
Y.Igarashi
(2003).
Characterization of a bifunctional archaeal acyl coenzyme A carboxylase.
|
| |
J Bacteriol,
185,
938-947.
|
 |
|
|
|
|
 |
J.E.Cronan
(2002).
Interchangeable enzyme modules. Functional replacement of the essential linker of the biotinylated subunit of acetyl-CoA carboxylase with a linker from the lipoylated subunit of pyruvate dehydrogenase.
|
| |
J Biol Chem,
277,
22520-22527.
|
 |
|
|
|
|
 |
J.E.Cronan,
and
G.L.Waldrop
(2002).
Multi-subunit acetyl-CoA carboxylases.
|
| |
Prog Lipid Res,
41,
407-435.
|
 |
|
|
|
|
 |
J.Solbiati,
A.Chapman-Smith,
and
J.E.Cronan
(2002).
Stabilization of the biotinoyl domain of Escherichia coli acetyl-CoA carboxylase by interactions between the attached biotin and the protruding "thumb" structure.
|
| |
J Biol Chem,
277,
21604-21609.
|
 |
|
|
|
|
 |
L.H.Weaver,
K.Kwon,
D.Beckett,
and
B.W.Matthews
(2001).
Competing protein:protein interactions are proposed to control the biological switch of the E coli biotin repressor.
|
| |
Protein Sci,
10,
2618-2622.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.D.Jones,
K.M.Stott,
M.J.Howard,
and
R.N.Perham
(2000).
Restricted motion of the lipoyl-lysine swinging arm in the pyruvate dehydrogenase complex of Escherichia coli.
|
| |
Biochemistry,
39,
8448-8459.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.N.Perham
(2000).
Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions.
|
| |
Annu Rev Biochem,
69,
961.
|
 |
|
|
|
|
 |
A.Chapman-Smith,
and
J.E.Cronan
(1999).
The enzymatic biotinylation of proteins: a post-translational modification of exceptional specificity.
|
| |
Trends Biochem Sci,
24,
359-363.
|
 |
|
|
|
|
 |
A.Chapman-Smith,
T.W.Morris,
J.C.Wallace,
and
J.E.Cronan
(1999).
Molecular recognition in a post-translational modification of exceptional specificity. Mutants of the biotinylated domain of acetyl-CoA carboxylase defective in recognition by biotin protein ligase.
|
| |
J Biol Chem,
274,
1449-1457.
|
 |
|
|
|
|
 |
C.Z.Blanchard,
A.Chapman-Smith,
J.C.Wallace,
and
G.L.Waldrop
(1999).
The biotin domain peptide from the biotin carboxyl carrier protein of Escherichia coli acetyl-CoA carboxylase causes a marked increase in the catalytic efficiency of biotin carboxylase and carboxyltransferase relative to free biotin.
|
| |
J Biol Chem,
274,
31767-31769.
|
 |
|
|
|
|
 |
D.Beckett,
E.Kovaleva,
and
P.J.Schatz
(1999).
A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation.
|
| |
Protein Sci,
8,
921-929.
|
 |
|
|
|
|
 |
X.Yao,
C.Soden,
M.F.Summers,
and
D.Beckett
(1999).
Comparison of the backbone dynamics of the apo- and holo-carboxy-terminal domain of the biotin carboxyl carrier subunit of Escherichia coli acetyl-CoA carboxylase.
|
| |
Protein Sci,
8,
307-317.
|
 |
|
|
|
|
 |
D.V.Reddy,
S.Rothemund,
B.C.Shenoy,
P.R.Carey,
and
F.D.Sönnichsen
(1998).
Structural characterization of the entire 1.3S subunit of transcarboxylase from Propionibacterium shermanii.
|
| |
Protein Sci,
7,
2156-2163.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

