
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase/transferase
|
 |
|
Title:
|
 |
Nizn[fe4s4] and nini[fe4s4] clusters in closed and open alpha subunits of acetyl-coa synthase/carbon monoxide dehydrogenase
|
|
Structure:
|
 |
Carbon monoxide dehydrogenase/acetyl-coa synthase subunit beta. Chain: a, b. Synonym: codh. Carbon monoxide dehydrogenase/acetyl-coa synthase subunit alpha. Chain: c, d. Synonym: codh. Ec: 2.3.1.169
|
|
Source:
|
 |
Moorella thermoacetica. Organism_taxid: 1525. Organism_taxid: 1525
|
|
Biol. unit:
|
 |
Tetramer (from PDB file)
|
|
Resolution:
|
 |
|
1.90Å
|
R-factor:
|
0.149
|
R-free:
|
0.179
|
|
|
Authors:
|
 |
C.Darnault,A.Volbeda,E.J.Kim,P.Legrand,X.Vernede,P.A.Lindahl, J.C.Fontecilla-Camps
|
Key ref:

|
 |
C.Darnault
et al.
(2003).
Ni-Zn-[Fe4-S4] and Ni-Ni-[Fe4-S4] clusters in closed and open subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase.
Nat Struct Biol,
10,
271-279.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
20-Jan-03
|
Release date:
|
11-Apr-03
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B:
E.C.1.2.7.4
- anaerobic carbon-monoxide dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
CO + 2 oxidized [2Fe-2S]-[ferredoxin] + H2O = 2 reduced [2Fe-2S]- [ferredoxin] + CO2 + 2 H+
|
 |
 |
 |
 |
 |
CO
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
2
×
oxidized [2Fe-2S]-[ferredoxin]
|
+
|
H2O
|
=
|
2
×
reduced [2Fe-2S]- [ferredoxin]
|
+
|
CO2
Bound ligand (Het Group name = )
matches with 75.00% similarity
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe cation; Ni(2+); Zn(2+)
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains C, D:
E.C.2.3.1.169
- CO-methylating acetyl-CoA synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Co(I)-[corrinoid Fe-S protein] + acetyl-CoA + H+ = methyl-Co(III)- [corrinoid Fe-S protein] + CO + CoA
|
 |
 |
 |
 |
 |
Co(I)-[corrinoid Fe-S protein]
|
+
|
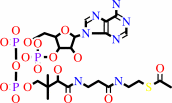 2
×
acetyl-CoA
2
×
acetyl-CoA
|
+
|
H(+)
|
=
|
2
×
methyl-Co(III)- [corrinoid Fe-S protein]
|
+
|
CO
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
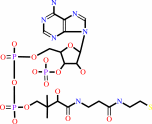 2
×
CoA
2
×
CoA
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Cu(2+); Iron-sulfur; Ni(2+)
|
 |
 |
 |
 |
 |
Cu(2+)
|
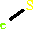 Iron-sulfur
Iron-sulfur
|
Ni(2+)
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Struct Biol
10:271-279
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Ni-Zn-[Fe4-S4] and Ni-Ni-[Fe4-S4] clusters in closed and open subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase.
|
|
C.Darnault,
A.Volbeda,
E.J.Kim,
P.Legrand,
X.Vernède,
P.A.Lindahl,
J.C.Fontecilla-Camps.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of the tetrameric alpha2beta2 acetyl-coenzyme A
synthase/carbon monoxide dehydrogenase from Moorella thermoacetica has been
solved at 1.9 A resolution. Surprisingly, the two alpha subunits display
different (open and closed) conformations. Furthermore, X-ray data collected
from crystals near the absorption edges of several metal ions indicate that the
closed form contains one Zn and one Ni at its active site metal cluster
(A-cluster) in the alpha subunit, whereas the open form has two Ni ions at the
corresponding positions. Alternative metal contents at the active site have been
observed in a recent structure of the same protein in which A-clusters contained
one Cu and one Ni, and in reconstitution studies of a recombinant apo form of a
related acetyl-CoA synthase. On the basis of our observations along with
previously reported data, we postulate that only the A-clusters containing two
Ni ions are catalytically active.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Stereo view of a superposition of the A[o]- and
A[c]-clusters. The coordination of Ni[p] in the A[o] cluster
(atoms color-coded as in Fig. 2) indicates a square planar
arrangement with three cysteine thiolates and one unidentified
exogenous ligand (L). In the A[c]-cluster (gray), the
coordination of the proximal Zn ion is distorted tetrahedral
(see also Fig. 5b). A 7  electron
density peak is found in a mF[o] - F[c] map that is 1.3 Å
removed from Ni[p] and that occupies, as shown, a position
equivalent to the Zn in the A[c]-cluster. electron
density peak is found in a mF[o] - F[c] map that is 1.3 Å
removed from Ni[p] and that occupies, as shown, a position
equivalent to the Zn in the A[c]-cluster.
|
 |
Figure 4.
Figure 4. Stereo views of the structure of the C-cluster in the
 subunit.
a, Electron density, contoured at the 1 subunit.
a, Electron density, contoured at the 1  level,
corresponding to a 2mF[o] - DF[c] difference electron density
map42 using 1.9 Å resolution refined model phases of CO-treated
crystal CO[a]. b, Residual mF[o] - F[c] electron density peaks,
contoured at the 5.5 level,
corresponding to a 2mF[o] - DF[c] difference electron density
map42 using 1.9 Å resolution refined model phases of CO-treated
crystal CO[a]. b, Residual mF[o] - F[c] electron density peaks,
contoured at the 5.5  level,
correponding to (i) a putative persulfide formed between a
labile S atom and Cys316, (ii) an alternative position for the
unique Fe, as indicated by arrows and (iii) alternative
conformations of Cys550. Atoms at alternative positions are
shown as translucid spheres. Atoms are colored as in Fig. 2. level,
correponding to (i) a putative persulfide formed between a
labile S atom and Cys316, (ii) an alternative position for the
unique Fe, as indicated by arrows and (iii) alternative
conformations of Cys550. Atoms at alternative positions are
shown as translucid spheres. Atoms are colored as in Fig. 2.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Struct Biol
(2003,
10,
271-279)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Krissinel
(2011).
Macromolecular complexes in crystals and solutions.
|
| |
Acta Crystallogr D Biol Crystallogr,
67,
376-385.
|
 |
|
|
|
|
 |
Y.Kung,
and
C.L.Drennan
(2011).
A role for nickel-iron cofactors in biological carbon monoxide and carbon dioxide utilization.
|
| |
Curr Opin Chem Biol,
15,
276-283.
|
 |
|
|
|
|
 |
Y.Liu,
X.Zhu,
F.Wang,
T.Ying,
P.Li,
Z.X.Huang,
and
X.Tan
(2011).
Probing the role of the bridging C509 between the [Fe4S4] cubane and the [Ni(p)Ni(d)] centre in the A-cluster of acetyl-coenzyme A synthase.
|
| |
Chem Commun (Camb),
47,
1291-1293.
|
 |
|
|
|
|
 |
C.Núñez,
R.Bastida,
A.Macías,
L.Valencia,
J.Ribas,
J.L.Capelo,
and
C.Lodeiro
(2010).
New dinuclear nickel(II) and iron(II) complexes with a macrocyclic ligand containing a N6S2 donor-set: synthesis, structural, MALDI-TOF-MS, magnetic and spectroscopic studies.
|
| |
Dalton Trans,
39,
7673-7683.
|
 |
|
|
|
|
 |
K.C.Ryan,
O.E.Johnson,
D.E.Cabelli,
T.C.Brunold,
and
M.J.Maroney
(2010).
Nickel superoxide dismutase: structural and functional roles of Cys2 and Cys6.
|
| |
J Biol Inorg Chem,
15,
795-807.
|
 |
|
|
|
|
 |
T.Matsumoto,
M.Ito,
M.Kotera,
and
K.Tatsumi
(2010).
A dinuclear nickel complex modeling of the Ni(d)(II)-Ni(p)(I) state of the active site of acetyl CoA synthase.
|
| |
Dalton Trans,
39,
2995-2997.
|
 |
|
|
|
|
 |
A.Panja,
C.Campana,
C.Leavitt,
M.J.Van Stipdonk,
and
D.M.Eichhorn
(2009).
Iron and Cobalt Complexes of 2,6-Diacetylpyridine-bis(R-thiosemicarbazone) (R=H, phenyl) Showing Unprecedented Ligand Deviation from Planarity.
|
| |
Inorganica Chim Acta,
362,
1348-1354.
|
 |
|
|
|
|
 |
A.Volbeda,
C.Darnault,
X.Tan,
P.A.Lindahl,
and
J.C.Fontecilla-Camps
(2009).
Novel domain arrangement in the crystal structure of a truncated acetyl-CoA synthase from Moorella thermoacetica.
|
| |
Biochemistry,
48,
7916-7926.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.C.Fontecilla-Camps,
P.Amara,
C.Cavazza,
Y.Nicolet,
and
A.Volbeda
(2009).
Structure-function relationships of anaerobic gas-processing metalloenzymes.
|
| |
Nature,
460,
814-822.
|
 |
|
|
|
|
 |
K.N.Green,
S.M.Brothers,
B.Lee,
M.Y.Darensbourg,
and
D.A.Rockcliffe
(2009).
Chemical issues addressing the construction of the distal Ni[cysteine-glycine-cysteine]2- site of acetyl CoA synthase: why not copper?
|
| |
Inorg Chem,
48,
2780-2792.
|
 |
|
|
|
|
 |
M.Ito,
M.Kotera,
T.Matsumoto,
and
K.Tatsumi
(2009).
Dinuclear nickel complexes modeling the structure and function of the acetyl CoA synthase active site.
|
| |
Proc Natl Acad Sci U S A,
106,
11862-11866.
|
 |
|
|
|
|
 |
N.Volkmann
(2009).
Confidence intervals for fitting of atomic models into low-resolution densities.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
679-689.
|
 |
|
|
|
|
 |
S.W.Ragsdale
(2009).
Nickel-based Enzyme Systems.
|
| |
J Biol Chem,
284,
18571-18575.
|
 |
|
|
|
|
 |
Y.Kung,
T.I.Doukov,
J.Seravalli,
S.W.Ragsdale,
and
C.L.Drennan
(2009).
Crystallographic snapshots of cyanide- and water-bound C-clusters from bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase.
|
| |
Biochemistry,
48,
7432-7440.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.Aragão,
E.P.Mitchell,
C.F.Frazão,
M.A.Carrondo,
and
P.F.Lindley
(2008).
Structural and functional relationships in the hybrid cluster protein family: structure of the anaerobically purified hybrid cluster protein from Desulfovibrio vulgaris at 1.35 A resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
665-674.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.L.Drake,
A.S.Gössner,
and
S.L.Daniel
(2008).
Old acetogens, new light.
|
| |
Ann N Y Acad Sci,
1125,
100-128.
|
 |
|
|
|
|
 |
J.Seravalli,
and
S.W.Ragsdale
(2008).
Pulse-chase studies of the synthesis of acetyl-CoA by carbon monoxide dehydrogenase/acetyl-CoA synthase: evidence for a random mechanism of methyl and carbonyl addition.
|
| |
J Biol Chem,
283,
8384-8394.
|
 |
|
|
|
|
 |
J.Seravalli,
and
S.W.Ragsdale
(2008).
13C NMR characterization of an exchange reaction between CO and CO2 catalyzed by carbon monoxide dehydrogenase.
|
| |
Biochemistry,
47,
6770-6781.
|
 |
|
|
|
|
 |
L.G.Trabuco,
E.Villa,
K.Mitra,
J.Frank,
and
K.Schulten
(2008).
Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics.
|
| |
Structure,
16,
673-683.
|
 |
|
|
|
|
 |
S.W.Ragsdale
(2008).
Enzymology of the wood-Ljungdahl pathway of acetogenesis.
|
| |
Ann N Y Acad Sci,
1125,
129-136.
|
 |
|
|
|
|
 |
S.W.Ragsdale,
and
E.Pierce
(2008).
Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation.
|
| |
Biochim Biophys Acta,
1784,
1873-1898.
|
 |
|
|
|
|
 |
S.W.Ragsdale
(2008).
Catalysis of methyl group transfers involving tetrahydrofolate and B(12).
|
| |
Vitam Horm,
79,
293-324.
|
 |
|
|
|
|
 |
T.I.Doukov,
L.C.Blasiak,
J.Seravalli,
S.W.Ragsdale,
and
C.L.Drennan
(2008).
Xenon in and at the end of the tunnel of bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase.
|
| |
Biochemistry,
47,
3474-3483.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Gong,
B.Hao,
Z.Wei,
D.J.Ferguson,
T.Tallant,
J.A.Krzycki,
and
M.K.Chan
(2008).
Structure of the alpha2epsilon2 Ni-dependent CO dehydrogenase component of the Methanosarcina barkeri acetyl-CoA decarbonylase/synthase complex.
|
| |
Proc Natl Acad Sci U S A,
105,
9558-9563.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
X.Tan,
M.Martinho,
A.Stubna,
P.A.Lindahl,
and
E.Münck
(2008).
Mossbauer evidence for an exchange-coupled {[Fe4S4]1+ Nip1+} A-cluster in isolated alpha subunits of acetyl-coenzyme A synthase/carbon monoxide dehydrogenase.
|
| |
J Am Chem Soc,
130,
6712-6713.
|
 |
|
|
|
|
 |
X.Tan,
and
P.A.Lindahl
(2008).
Tunnel mutagenesis and Ni-dependent reduction and methylation of the alpha subunit of acetyl coenzyme A synthase/carbon monoxide dehydrogenase.
|
| |
J Biol Inorg Chem,
13,
771-778.
|
 |
|
|
|
|
 |
Y.Ohki,
Y.Takikawa,
H.Sadohara,
C.Kesenheimer,
B.Engendahl,
E.Kapatina,
and
K.Tatsumi
(2008).
Reactions at the Ru-S bonds of coordinatively unsaturated ruthenium complexes with tethered 2,6-dimesitylphenyl thiolate.
|
| |
Chem Asian J,
3,
1625-1635.
|
 |
|
|
|
|
 |
B.E.Mann,
and
R.Motterlini
(2007).
CO and NO in medicine.
|
| |
Chem Commun (Camb),
(),
4197-4208.
|
 |
|
|
|
|
 |
B.J.Johnson,
J.Cohen,
R.W.Welford,
A.R.Pearson,
K.Schulten,
J.P.Klinman,
and
C.M.Wilmot
(2007).
Exploring molecular oxygen pathways in Hansenula polymorpha copper-containing amine oxidase.
|
| |
J Biol Chem,
282,
17767-17776.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.W.Smucker,
M.J.Vanstipdonk,
and
D.M.Eichhorn
(2007).
Incorporation of thiolate donation using 2,2'-dithiodibenzaldehyde: complexes of a pentadentate N2S3 ligand with relevance to the active site of Co nitrile hydratase.
|
| |
J Inorg Biochem,
101,
1537-1542.
|
 |
|
|
|
|
 |
J.H.Jeoung,
and
H.Dobbek
(2007).
Carbon dioxide activation at the Ni,Fe-cluster of anaerobic carbon monoxide dehydrogenase.
|
| |
Science,
318,
1461-1464.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.J.Russell
(2007).
The alkaline solution to the emergence of life: energy, entropy and early evolution.
|
| |
Acta Biotheor,
55,
133-179.
|
 |
|
|
|
|
 |
S.W.Ha,
M.Korbas,
M.Klepsch,
W.Meyer-Klaucke,
O.Meyer,
and
V.Svetlitchnyi
(2007).
Interaction of potassium cyanide with the [Ni-4Fe-5S] active site cluster of CO dehydrogenase from Carboxydothermus hydrogenoformans.
|
| |
J Biol Chem,
282,
10639-10646.
|
 |
|
|
|
|
 |
S.W.Ragsdale
(2007).
Nickel and the carbon cycle.
|
| |
J Inorg Biochem,
101,
1657-1666.
|
 |
|
|
|
|
 |
W.Martin,
and
M.J.Russell
(2007).
On the origin of biochemistry at an alkaline hydrothermal vent.
|
| |
Philos Trans R Soc Lond B Biol Sci,
362,
1887-1925.
|
 |
|
|
|
|
 |
P.M.Rodrigues,
A.L.Macedo,
B.J.Goodfellow,
I.Moura,
and
J.J.Moura
(2006).
Desulfovibrio gigas ferredoxin II: redox structural modulation of the [3Fe-4S] cluster.
|
| |
J Biol Inorg Chem,
11,
307-315.
|
 |
|
|
|
|
 |
T.A.Stich,
J.Seravalli,
S.Venkateshrao,
T.G.Spiro,
S.W.Ragsdale,
and
T.C.Brunold
(2006).
Spectroscopic studies of the corrinoid/iron-sulfur protein from Moorella thermoacetica.
|
| |
J Am Chem Soc,
128,
5010-5020.
|
 |
|
|
|
|
 |
X.Tan,
A.Volbeda,
J.C.Fontecilla-Camps,
and
P.A.Lindahl
(2006).
Function of the tunnel in acetylcoenzyme A synthase/carbon monoxide dehydrogenase.
|
| |
J Biol Inorg Chem,
11,
371-378.
|
 |
|
|
|
|
 |
X.Tan,
I.V.Surovtsev,
and
P.A.Lindahl
(2006).
Kinetics of CO insertion and acetyl group transfer steps, and a model of the acetyl-CoA synthase catalytic mechanism.
|
| |
J Am Chem Soc,
128,
12331-12338.
|
 |
|
|
|
|
 |
A.Meyerdierks,
M.Kube,
T.Lombardot,
K.Knittel,
M.Bauer,
F.O.Glöckner,
R.Reinhardt,
and
R.Amann
(2005).
Insights into the genomes of archaea mediating the anaerobic oxidation of methane.
|
| |
Environ Microbiol,
7,
1937-1951.
|
 |
|
|
|
|
 |
A.Volbeda,
and
J.C.Fontecilla-Camps
(2005).
Structural bases for the catalytic mechanism of Ni-containing carbon monoxide dehydrogenases.
|
| |
Dalton Trans,
(),
3443-3450.
|
 |
|
|
|
|
 |
D.A.Grahame,
S.Gencic,
and
E.DeMoll
(2005).
A single operon-encoded form of the acetyl-CoA decarbonylase/synthase multienzyme complex responsible for synthesis and cleavage of acetyl-CoA in Methanosarcina thermophila.
|
| |
Arch Microbiol,
184,
32-40.
|
 |
|
|
|
|
 |
D.C.Johnson,
D.R.Dean,
A.D.Smith,
and
M.K.Johnson
(2005).
Structure, function, and formation of biological iron-sulfur clusters.
|
| |
Annu Rev Biochem,
74,
247-281.
|
 |
|
|
|
|
 |
M.V.Rampersad,
S.P.Jeffery,
J.H.Reibenspies,
C.G.Ortiz,
D.J.Darensbourg,
and
M.Y.Darensbourg
(2005).
N2S2Ni metallothiolates as a class of ligands that support organometallic and bioorganometallic reactivity.
|
| |
Angew Chem Int Ed Engl,
44,
1217-1220.
|
 |
|
|
|
|
 |
R.Panda,
C.P.Berlinguette,
Y.Zhang,
and
R.H.Holm
(2005).
Synthesis of MFe3S4 clusters containing a planar M(II) site (M = Ni, Pd, Pt), a structural element in the C-cluster of carbon monoxide dehydrogenase.
|
| |
J Am Chem Soc,
127,
11092-11101.
|
 |
|
|
|
|
 |
S.L.Andrade,
F.Cruz,
C.L.Drennan,
V.Ramakrishnan,
D.C.Rees,
J.G.Ferry,
and
O.Einsle
(2005).
Structures of the iron-sulfur flavoproteins from Methanosarcina thermophila and Archaeoglobus fulgidus.
|
| |
J Bacteriol,
187,
3848-3854.
|
 |
|
|
|
|
 |
W.B.Jeon,
S.W.Singer,
P.W.Ludden,
and
L.M.Rubio
(2005).
New insights into the mechanism of nickel insertion into carbon monoxide dehydrogenase: analysis of Rhodospirillum rubrum carbon monoxide dehydrogenase variants with substituted ligands to the [Fe3S4] portion of the active-site C-cluster.
|
| |
J Biol Inorg Chem,
10,
903-912.
|
 |
|
|
|
|
 |
W.Zhu,
A.C.Marr,
Q.Wang,
F.Neese,
D.J.Spencer,
A.J.Blake,
P.A.Cooke,
C.Wilson,
and
M.Schröder
(2005).
Modulation of the electronic structure and the Ni-Fe distance in heterobimetallic models for the active site in [NiFe]hydrogenase.
|
| |
Proc Natl Acad Sci U S A,
102,
18280-18285.
|
 |
|
|
|
|
 |
B.M.Martins,
H.Dobbek,
I.Cinkaya,
W.Buckel,
and
A.Messerschmidt
(2004).
Crystal structure of 4-hydroxybutyryl-CoA dehydratase: radical catalysis involving a [4Fe-4S] cluster and flavin.
|
| |
Proc Natl Acad Sci U S A,
101,
15645-15649.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.P.Roberts,
H.Youn,
and
R.L.Kerby
(2004).
CO-sensing mechanisms.
|
| |
Microbiol Mol Biol Rev,
68,
453-473.
|
 |
|
|
|
|
 |
J.Wuerges,
J.W.Lee,
Y.I.Yim,
H.S.Yim,
S.O.Kang,
and
K.Djinovic Carugo
(2004).
Crystal structure of nickel-containing superoxide dismutase reveals another type of active site.
|
| |
Proc Natl Acad Sci U S A,
101,
8569-8574.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Gerstein,
and
N.Echols
(2004).
Exploring the range of protein flexibility, from a structural proteomics perspective.
|
| |
Curr Opin Chem Biol,
8,
14-19.
|
 |
|
|
|
|
 |
M.J.Russell,
and
W.Martin
(2004).
The rocky roots of the acetyl-CoA pathway.
|
| |
Trends Biochem Sci,
29,
358-363.
|
 |
|
|
|
|
 |
V.Svetlitchnyi,
H.Dobbek,
W.Meyer-Klaucke,
T.Meins,
B.Thiele,
P.Römer,
R.Huber,
and
O.Meyer
(2004).
A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans.
|
| |
Proc Natl Acad Sci U S A,
101,
446-451.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.C.Rees,
and
J.B.Howard
(2003).
The interface between the biological and inorganic worlds: iron-sulfur metalloclusters.
|
| |
Science,
300,
929-931.
|
 |
|
|
|
|
 |
J.Chen,
S.Huang,
J.Seravalli,
H.Gutzman,
D.J.Swartz,
S.W.Ragsdale,
and
K.A.Bagley
(2003).
Infrared studies of carbon monoxide binding to carbon monoxide dehydrogenase/acetyl-CoA synthase from Moorella thermoacetica.
|
| |
Biochemistry,
42,
14822-14830.
|
 |
|
|
|
|
 |
P.D.Barker
(2003).
Designing redox metalloproteins from bottom-up and top-down perspectives.
|
| |
Curr Opin Struct Biol,
13,
490-499.
|
 |
|
|
|
|
 |
R.P.Hausinger
(2003).
Ni and CO: more surprises.
|
| |
Nat Struct Biol,
10,
234-236.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

