 |
PDBsum entry 3b51

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
3b51
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.2.7.4
- anaerobic carbon-monoxide dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
CO + 2 oxidized [2Fe-2S]-[ferredoxin] + H2O = 2 reduced [2Fe-2S]- [ferredoxin] + CO2 + 2 H+
|
 |
 |
 |
 |
 |
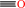 CO
CO
|
+
|
2
×
oxidized [2Fe-2S]-[ferredoxin]
|
+
|
H2O
|
=
|
2
×
reduced [2Fe-2S]- [ferredoxin]
|
+
|
 CO2
CO2
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe cation; Ni(2+); Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Science
318:1461-1464
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Carbon dioxide activation at the Ni,Fe-cluster of anaerobic carbon monoxide dehydrogenase.
|
|
J.H.Jeoung,
H.Dobbek.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Anaerobic CO dehydrogenases catalyze the reversible oxidation of CO to CO2 at a
complex Ni-, Fe-, and S-containing metal center called cluster C. We report
crystal structures of CO dehydrogenase II from Carboxydothermus hydrogenoformans
in three different states. In a reduced state, exogenous CO2 supplied in
solution is bound and reductively activated by cluster C. In the intermediate
structure, CO2 acts as a bridging ligand between Ni and the asymmetrically
coordinated Fe, where it completes the square-planar coordination of the Ni ion.
It replaces a water/hydroxo ligand bound to the Fe ion in the other two states.
The structures define the mechanism of CO oxidation and CO2 reduction at the
Ni-Fe site of cluster C.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. The –600 mV (A), –600 mV+CO[2] (B) and –320 mV
(C) states of cluster C. 2F[obs] – F[calc] maps in blue are
contoured at 1  , and F[obs] –
F[calc] maps in green are contoured at 4.5 , and F[obs] –
F[calc] maps in green are contoured at 4.5  . For the
calculation of the F[obs] – F[calc] map, the OH[x] ligand [(A)
and (C)] and the CO[2] ligand (B) have been removed from the
model. An alternative position found for Fe1, termed Fe1B, is
depicted in transparent light gray. The occupancies for the
alternative position have been estimated to 10 to 30%. Selected
distances are shown in Å. For more details on the geometry
of the three states, see figs. S2 to S4. All pictures were
prepared by using PyMol (23). . For the
calculation of the F[obs] – F[calc] map, the OH[x] ligand [(A)
and (C)] and the CO[2] ligand (B) have been removed from the
model. An alternative position found for Fe1, termed Fe1B, is
depicted in transparent light gray. The occupancies for the
alternative position have been estimated to 10 to 30%. Selected
distances are shown in Å. For more details on the geometry
of the three states, see figs. S2 to S4. All pictures were
prepared by using PyMol (23).
|
 |
Figure 2.
Fig. 2. Superposition of the –600 mV (blue) and –600
mV+CO[2] (element colors) states.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the AAAs:
Science
(2007,
318,
1461-1464)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.Huang,
O.V.Makhlynets,
L.L.Tan,
S.C.Lee,
E.V.Rybak-Akimova,
and
R.H.Holm
(2011).
Kinetics and mechanistic analysis of an extremely rapid carbon dioxide fixation reaction.
|
| |
Proc Natl Acad Sci U S A,
108,
1222-1227.
|
 |
|
|
|
|
 |
Y.Kung,
and
C.L.Drennan
(2011).
A role for nickel-iron cofactors in biological carbon monoxide and carbon dioxide utilization.
|
| |
Curr Opin Chem Biol,
15,
276-283.
|
 |
|
|
|
|
 |
D.Huang,
and
R.H.Holm
(2010).
Reactions of the terminal Ni(II)-OH group in substitution and electrophilic reactions with carbon dioxide and other substrates: structural definition of binding modes in an intramolecular Ni(II)...Fe(II) bridged site.
|
| |
J Am Chem Soc,
132,
4693-4701.
|
 |
|
|
|
|
 |
T.W.Woolerton,
S.Sheard,
E.Reisner,
E.Pierce,
S.W.Ragsdale,
and
F.A.Armstrong
(2010).
Efficient and clean photoreduction of CO(2) to CO by enzyme-modified TiO(2) nanoparticles using visible light.
|
| |
J Am Chem Soc,
132,
2132-2133.
|
 |
|
|
|
|
 |
A.Volbeda,
C.Darnault,
X.Tan,
P.A.Lindahl,
and
J.C.Fontecilla-Camps
(2009).
Novel domain arrangement in the crystal structure of a truncated acetyl-CoA synthase from Moorella thermoacetica.
|
| |
Biochemistry,
48,
7916-7926.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.E.Benson,
C.P.Kubiak,
A.J.Sathrum,
and
J.M.Smieja
(2009).
Electrocatalytic and homogeneous approaches to conversion of CO(2) to liquid fuels.
|
| |
Chem Soc Rev,
38,
89-99.
|
 |
|
|
|
|
 |
J.C.Fontecilla-Camps,
P.Amara,
C.Cavazza,
Y.Nicolet,
and
A.Volbeda
(2009).
Structure-function relationships of anaerobic gas-processing metalloenzymes.
|
| |
Nature,
460,
814-822.
|
 |
|
|
|
|
 |
M.T.Mock,
M.T.Kieber-Emmons,
C.V.Popescu,
P.Gasda,
G.P.Yap,
and
C.G.Riordan
(2009).
A Series of Cyanide-Bridged Binuclear Complexes.
|
| |
Inorganica Chim Acta,
362,
4553-4562.
|
 |
|
|
|
|
 |
S.Groysman,
and
R.H.Holm
(2009).
Biomimetic chemistry of iron, nickel, molybdenum, and tungsten in sulfur-ligated protein sites.
|
| |
Biochemistry,
48,
2310-2320.
|
 |
|
|
|
|
 |
Y.Kung,
T.I.Doukov,
J.Seravalli,
S.W.Ragsdale,
and
C.L.Drennan
(2009).
Crystallographic snapshots of cyanide- and water-bound C-clusters from bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase.
|
| |
Biochemistry,
48,
7432-7440.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Seravalli,
and
S.W.Ragsdale
(2008).
13C NMR characterization of an exchange reaction between CO and CO2 catalyzed by carbon monoxide dehydrogenase.
|
| |
Biochemistry,
47,
6770-6781.
|
 |
|
|
|
|
 |
K.Bates,
M.Wouldhave,
and
R.A.Henderson
(2008).
Involvement of thiolate ligands in binding substrates to Fe-S clusters.
|
| |
Dalton Trans,
(),
6527-6529.
|
 |
|
|
|
|
 |
S.W.Ragsdale,
and
E.Pierce
(2008).
Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation.
|
| |
Biochim Biophys Acta,
1784,
1873-1898.
|
 |
|
|
|
|
 |
W.G.Dougherty,
K.Rangan,
M.J.O'Hagan,
G.P.Yap,
and
C.G.Riordan
(2008).
Binuclear complexes containing a methylnickel moiety: relevance to organonickel intermediates in acetyl coenzyme A synthase catalysis.
|
| |
J Am Chem Soc,
130,
13510-13511.
|
 |
|
|
|
|
 |
W.Gong,
B.Hao,
Z.Wei,
D.J.Ferguson,
T.Tallant,
J.A.Krzycki,
and
M.K.Chan
(2008).
Structure of the alpha2epsilon2 Ni-dependent CO dehydrogenase component of the Methanosarcina barkeri acetyl-CoA decarbonylase/synthase complex.
|
| |
Proc Natl Acad Sci U S A,
105,
9558-9563.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

