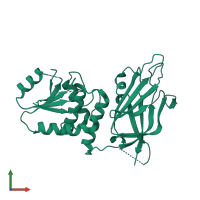
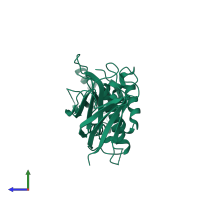
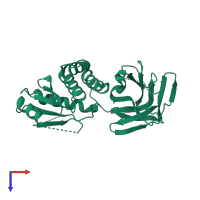
Function and Biology Details
Reactions catalysed:
[a protein]-serine/threonine phosphate + H(2)O = [a protein]-serine/threonine + phosphate
Protein tyrosine phosphate + H(2)O = protein tyrosine + phosphate
Phosphatidylinositol 3,4,5-trisphosphate + H(2)O = phosphatidylinositol 4,5-bisphosphate + phosphate
Biochemical function:
Biological process:
Cellular component:
- not assigned
Sequence domains:
- Tensin phosphatase, C2 domain
- Protein-tyrosine phosphatase-like
- Bifunctional phosphatidylinositol trisphosphate phosphatase/dual specificity phosphatase PTEN
- C2 domain superfamily
- Protein-tyrosine phosphatase, active site
- Tyrosine-specific protein phosphatases domain
- PTEN, phosphatase domain
- Protein-tyrosine phosphatase, catalytic
1 more domain
Structure analysis Details
Assembly composition:
monomeric (preferred)
Assembly name:
PDBe Complex ID:
PDB-CPX-158017 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule:
Ligands and Environments
No bound ligands
No modified residues
Experiments and Validation Details
X-ray source:
APS BEAMLINE 24-ID-E
Spacegroup:
I4
Expression system: Spodoptera frugiperda