 |
PDBsum entry 5e8l
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of geranylgeranyl pyrophosphate synthase 11 from arabidopsis thaliana
|
|
Structure:
|
 |
Heterodimeric geranylgeranyl pyrophosphate synthase large subunit 1, chloroplastic. Chain: a, b. Fragment: unp residues 72-371. Synonym: ggps1,(2e,6e)-farnesyl diphosphate synthase 1, dimethylallyltranstransferase 1,farnesyl diphosphate synthase 1, farnesyltranstransferase 1,geranyltranstransferase 1. Ec: 2.5.1.-,2.5.1.1,2.5.1.29,2.5.1.10. Engineered: yes
|
|
Source:
|
 |
Arabidopsis thaliana. Mouse-ear cress. Organism_taxid: 3702. Gene: ggpps1, ggpps11, ggps1, at4g36810, c7a10.550. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.81Å
|
R-factor:
|
0.191
|
R-free:
|
0.237
|
|
|
Authors:
|
 |
C.Wang,Q.Chen,D.Fan,J.Li,G.Wang,P.Zhang
|
|
Key ref:
|
 |
C.Wang
et al.
(2016).
Structural Analyses of Short-Chain Prenyltransferases Identify an Evolutionarily Conserved GFPPS Clade in Brassicaceae Plants.
Mol Plant,
9,
195-204.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
14-Oct-15
|
Release date:
|
11-Nov-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P34802
(GGPP1_ARATH) -
Heterodimeric geranylgeranyl pyrophosphate synthase large subunit 1, chloroplastic from Arabidopsis thaliana
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
371 a.a.
278 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.5.1.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.5.1.1
- dimethylallyltranstransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + dimethylallyl diphosphate = (2E)- geranyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
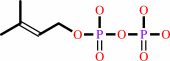 dimethylallyl diphosphate
dimethylallyl diphosphate
|
=
|
(2E)- geranyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.5.1.10
- (2E,6E)-farnesyl diphosphate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + (2E)-geranyl diphosphate = (2E,6E)-farnesyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
(2E)-geranyl diphosphate
|
=
|
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.2.5.1.29
- geranylgeranyl diphosphate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + (2E,6E)-farnesyl diphosphate = (2E,6E,10E)- geranylgeranyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
=
|
(2E,6E,10E)- geranylgeranyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Mol Plant
9:195-204
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural Analyses of Short-Chain Prenyltransferases Identify an Evolutionarily Conserved GFPPS Clade in Brassicaceae Plants.
|
|
C.Wang,
Q.Chen,
D.Fan,
J.Li,
G.Wang,
P.Zhang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Terpenoids are the largest and most diverse class of plant-specialized
metabolites, which function in diverse physiological processes during plant
development. In the biosynthesis of plant terpenoids, short-chain
prenyltransferases (SC-PTs), together with terpene synthases (TPSs), play
critical roles in determining terpenoid diversity. SC-PTs biosynthesize prenyl
pyrophosphates with different chain lengths, and these compounds are the direct
precursors of terpenoids. Arabidopsis thaliana possesses a subgroup of SC-PTs
whose functions are not clearly known. In this study, we focus on 10
geranylgeranyl pyrophosphate synthase-like [GGPPSL] proteins, which are commonly
thought to produce GGPP [C20]. We found that a subset of members of the
Arabidopsis GGPPSL gene family have undergone neo-functionalization: GGPPSL6, 7,
9, and 10 mainly have geranylfarnesyl pyrophosphate synthase activity (C25;
renamed AtGFPPS1, 2, 3, and 4), and GGPPSL8 produces even longer chain prenyl
pyrophosphate (≥ C30; renamed polyprenyl pyrophosphate synthase 2, AtPPPS2).
By solving the crystal structures of AtGFPPS2, AtPPPS2, and AtGGPPS11, we reveal
the product chain-length determination mechanism of SC-PTs and interpret it as a
"three floors" model. Using this model, we identified a novel GFPPS
clade distributed in Brassicaceae plants and found that the GFPPS gene typically
occurs in tandem with a gene encoding a TPS, forming a GFPPS-TPS gene cluster.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

