 |
PDBsum entry 2chm

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of n2 substituted pyrazolo pyrimidinones - a flipped binding mode in pde5
|
|
Structure:
|
 |
Cgmp-specific 3', 5'-cyclic phosphodiesterase, camp- specific 3', 5'-cyclic phosphodiesterase 4b. Chain: a. Fragment: catalytic domain residues (residues 534-858 with subdomain replaced with pde4 subdomain). Synonym: 3', 5' cgmp-cyclic phophodiesterase 5a catalytic domain chimera, cgb-pde, cgmp-binding cgmp-specific phosphodiesterase, dpde4, pde32. Engineered: yes.
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Expressed in: trichoplusia ni. Expression_system_taxid: 7111. Expression_system_cell_line: high five.
|
|
Resolution:
|
 |
|
1.60Å
|
R-factor:
|
0.195
|
R-free:
|
0.223
|
|
|
Authors:
|
 |
C.M.N.Allerton,C.G.Barber,K.C.Beaumont,D.G.Brown,S.M.Cole,D.Ellis, C.A.L.Lane,G.N.Maw,N.M.Mount,D.J.Rawson,C.M.Robinson,S.D.A.Street, N.W.Summerhill
|
|
Key ref:
|
 |
C.M.Allerton
et al.
(2006).
A novel series of potent and selective PDE5 inhibitors with potential for high and dose-independent oral bioavailability.
J Med Chem,
49,
3581-3594.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
15-Mar-06
|
Release date:
|
08-Jun-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.1.4.35
- 3',5'-cyclic-GMP phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3',5'-cyclic GMP + H2O = GMP + H+
|
 |
 |
 |
 |
 |
 3',5'-cyclic GMP
3',5'-cyclic GMP
|
+
|
H2O
|
=
|
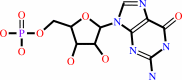 GMP
GMP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.1.4.53
- 3',5'-cyclic-AMP phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3',5'-cyclic AMP + H2O = AMP + H+
|
 |
 |
 |
 |
 |
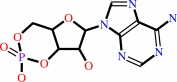 3',5'-cyclic AMP
3',5'-cyclic AMP
|
+
|
H2O
|
=
|
 AMP
AMP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
49:3581-3594
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
A novel series of potent and selective PDE5 inhibitors with potential for high and dose-independent oral bioavailability.
|
|
C.M.Allerton,
C.G.Barber,
K.C.Beaumont,
D.G.Brown,
S.M.Cole,
D.Ellis,
C.A.Lane,
G.N.Maw,
N.M.Mount,
D.J.Rawson,
C.M.Robinson,
S.D.Street,
N.W.Summerhill.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Sildenafil
(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one),
a potent and selective phosphodiesterase type 5 (PDE5) inhibitor, provided the
first oral treatment for male erectile dysfunction. The objective of the work
reported in this paper was to combine high levels of PDE5 potency and
selectivity with high and dose-independent oral bioavailability, to minimize the
impact on the C(max) of any interactions with coadministered drugs in the
clinic. This goal was achieved through identification of a lower clearance
series with a high absorption profile, by replacing the 5'-piperazine
sulfonamide in the sildenafil template with a 5'-methyl ketone. This novel
series provided compounds with low metabolism in human hepatocytes, excellent
caco-2 flux, and the potential for good aqueous solubility. The in vivo oral and
iv pharmacokinetic profiles of example compounds confirmed the high oral
bioavailability predicted from these in vitro screens.
5-(5-Acetyl-2-butoxy-3-pyridinyl)-3-ethyl-2-(1-ethyl-3-azetidinyl)-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one
(2) was selected for progression into the clinic.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.H.Hu,
and
M.H.Wu
(2008).
5-Butyl-amino-6-(4-fluoro-phen-yl)-7-oxo-1-p-tolyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidine-3-carbonitrile.
|
| |
Acta Crystallogr Sect E Struct Rep Online,
64,
o845.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |
|

