 |
PDBsum entry 2cg8

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Lyase/transferase
|
PDB id
|
|

|

|
2cg8
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lyase/transferase
|
 |
|
Title:
|
 |
The bifunctional dihydroneopterin aldolase 6-hydroxymethyl-7,8- dihydropterin synthase from streptococcus pneumoniae
|
|
Structure:
|
 |
Dihydroneopterin aldolase 6-hydroxymethyl-7,8-dihydropterin synthase. Chain: a, b, c, d. Synonym: dihydroneopterin aldolase, dhna, 2-amino-4-hydroxy-6- hydroxymethyldihydropteridine pyrophosphokinase, 7,8-dihydro-6- hydroxymethylpterin pyrophosphokinase, 6-hydroxymethyl-7,8- dihydropterin pyrophosphokinase, hppk, pppk. Engineered: yes
|
|
Source:
|
 |
Streptococcus pneumoniae. Organism_taxid: 1313. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Biol. unit:
|
 |
 Octamer (from PDB file)
Octamer (from PDB file)
|
|
Resolution:
|
 |
|
2.90Å
|
R-factor:
|
0.216
|
R-free:
|
0.241
|
|
|
Authors:
|
 |
A.Garcon,C.Levy,J.P.Derrick
|
Key ref:

|
 |
A.Garçon
et al.
(2006).
Crystal structure of the bifunctional dihydroneopterin aldolase/6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase from Streptococcus pneumoniae.
J Mol Biol,
360,
644-653.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
28-Feb-06
|
Release date:
|
21-Jun-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
Chains A, B, C, D:
E.C.2.7.6.3
- 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Folate Biosynthesis (late stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
6-hydroxymethyl-7,8-dihydropterin + ATP = (7,8-dihydropterin-6-yl)methyl diphosphate + AMP + H+
|
 |
 |
 |
 |
 |
6-hydroxymethyl-7,8-dihydropterin
|
+
|
 ATP
ATP
|
=
|
(7,8-dihydropterin-6-yl)methyl diphosphate
|
+
|
 AMP
AMP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+)
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, C, D:
E.C.4.1.2.25
- dihydroneopterin aldolase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
7,8-dihydroneopterin = 6-hydroxymethyl-7,8-dihydropterin + glycolaldehyde
|
 |
 |
 |
 |
 |
7,8-dihydroneopterin
|
=
|
6-hydroxymethyl-7,8-dihydropterin
|
+
|
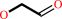 glycolaldehyde
glycolaldehyde
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
360:644-653
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of the bifunctional dihydroneopterin aldolase/6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase from Streptococcus pneumoniae.
|
|
A.Garçon,
C.Levy,
J.P.Derrick.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The enzymes dihydroneopterin aldolase (DHNA) and
6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase (HPPK) catalyse two
consecutive steps in the biosynthesis of folic acid. Neither of these enzymes
has a counterpart in mammals, and they have therefore been suggested as ideal
targets for antimicrobial drugs. Some of the enzymes within the folate pathway
can occur as bi- or trifunctional complexes in bacteria and parasites, but the
way in which bifunctional DHNA-HPPK enzymes are assembled is unclear. Here, we
report the determination of the structure at 2.9 A resolution of the DHNA-HPPK
(SulD) bifunctional enzyme complex from the respiratory pathogen Streptococcus
pneumoniae. In the crystal, DHNA is assembled as a core octamer, with 422 point
group symmetry, although the enzyme is active as a tetramer in solution.
Individual HPPK monomers are arranged at the ends of the DHNA octamer, making
relatively few contacts with the DHNA domain, but more extensive interactions
with adjacent HPPK domains. As a result, the structure forms an elongated
cylinder, with the HPPK domains forming two tetramers at each end. The active
sites of both enzymes face outward, and there is no clear channel between them
that could be used for channelling substrates. The HPPK-HPPK interface accounts
for about one-third of the total area between adjacent monomers in SulD, and has
levels of surface complementarity comparable to that of the DHNA-DHNA
interfaces. There is no "linker" polypeptide between DHNA and HPPK,
reducing the conformational flexibility of the HPPK domain relative to the DHNA
domain. The implications for the organisation of bi- and trifunctional enzyme
complexes within the folate biosynthesis pathway are discussed.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Reactions catalysed by DHNA, HPPK and DHPS.
|
 |
Figure 4.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2006,
360,
644-653)
copyright 2006.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Blaszczyk,
Y.Li,
J.Gan,
H.Yan,
and
X.Ji
(2007).
Structural basis for the aldolase and epimerase activities of Staphylococcus aureus dihydroneopterin aldolase.
|
| |
J Mol Biol,
368,
161-169.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Blaszczyk,
Y.Li,
S.Cherry,
J.Alexandratos,
Y.Wu,
G.Shaw,
J.E.Tropea,
D.S.Waugh,
H.Yan,
and
X.Ji
(2007).
Structure and activity of Yersinia pestis 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase as a novel target for the development of antiplague therapeutics.
|
| |
Acta Crystallogr D Biol Crystallogr,
63,
1169-1177.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |
|

