 |
PDBsum entry 1ynu

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.1.4.-.-
|
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.4.4.1.14
- 1-aminocyclopropane-1-carboxylate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
S-adenosyl-L-methionine = 1-aminocyclopropane-1-carboxylate + S-methyl- 5'-thioadenosine + H+
|
 |
 |
 |
 |
 |
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
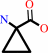 1-aminocyclopropane-1-carboxylate
1-aminocyclopropane-1-carboxylate
|
+
|
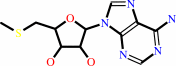 S-methyl- 5'-thioadenosine
S-methyl- 5'-thioadenosine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
 Pyridoxal 5'-phosphate
Pyridoxal 5'-phosphate
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
FEBS Lett
579:2458-2462
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of ACC synthase inactivated by the mechanism-based inhibitor L-vinylglycine.
|
|
G.Capitani,
M.Tschopp,
A.C.Eliot,
J.F.Kirsch,
M.G.Grütter.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
L-Vinylglycine (L-VG) is both a substrate for and a mechanism-based inhibitor of
1-aminocyclopropane-1-carboxylate (ACC) synthase. The ratio of the rate
constants for catalytic conversion to alpha-ketobutyrate and ammonia to
inactivation is 500/1. The crystal structure of the covalent adduct of the
inactivated enzyme was determined at 2.25 Angstroms resolution. The active site
contains an external aldimine of the adduct of L-VG with the pyridoxal
5'-phosphate cofactor. The side chain gamma-carbon of L-VG is covalently bound
to the epsilon-amino group of Lys273. This species corresponds to one of the two
alternatives proposed by Feng and Kirsch [Feng, L. and Kirsch, J.F. (2000)
L-Vinylglycine is an alternative substrate as well as a mechanism-based
inhibitor of 1-aminocyclopropane-1-carboxylate synthase. Biochemistry 39,
and presumably results from Michael addition to a vinylglycine
ketimine intermediate.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Schematic summary of the reactions of ACC synthase
with the inhibitors AVG and AMA. Prepared with ChemDraw.
|
 |
Figure 2.
Fig. 2. Schematic summary of the ACC synthase-catalyzed
reactions with the natural substrate, SAM and with l-VG.
Prepared with ChemDraw.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the Federation of European Biochemical Societies:
FEBS Lett
(2005,
579,
2458-2462)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.H.Küchenthal,
J.Migenda,
M.Polednia,
and
W.Maison
(2010).
An improved protocol for the preparation of (S)-vinylglycine from (S)-methionine.
|
| |
Amino Acids,
39,
443-448.
|
 |
|
|
|
|
 |
D.B.Berkowitz,
K.R.Karukurichi,
R.de la Salud-Bea,
D.L.Nelson,
and
C.D.McCune
(2008).
Use of Fluorinated Functionality in Enzyme Inhibitor Development: Mechanistic and Analytical Advantages.
|
| |
J Fluor Chem,
129,
731-742.
|
 |
|
|
|
|
 |
D.B.Berkowitz,
B.Wu,
and
H.Li
(2006).
A formal [3,3]-sigmatropic rearrangement route to quaternary alpha-vinyl amino acids: use of allylic N-PMP trifluoroacetimidates.
|
| |
Org Lett,
8,
971-974.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

