 |
PDBsum entry 1zg9

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of 5-{[amino(imino)methyl]amino}-2-(sulfanylmethyl) pentanoic acid bound to activated porcine pancreatic carboxypeptidase b
|
|
Structure:
|
 |
Procarboxypeptidase b. Chain: a, b, c. Fragment: catalytic domain. Ec: 3.4.17.2
|
|
Source:
|
 |
Sus scrofa. Pig. Organism_taxid: 9823
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.203
|
R-free:
|
0.272
|
|
|
Authors:
|
 |
M.Adler,J.Bryant,B.Buckman,I.Islam,B.Larsen,S.Finster,L.Kent,K.May, R.Mohan,S.Yuan,M.Whitlow
|
Key ref:

|
 |
M.Adler
et al.
(2005).
Crystal structures of potent thiol-based inhibitors bound to carboxypeptidase B.
Biochemistry,
44,
9339-9347.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
20-Apr-05
|
Release date:
|
12-Jul-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P09955
(CBPB1_PIG) -
Carboxypeptidase B from Sus scrofa
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
416 a.a.
305 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.17.2
- carboxypeptidase B.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
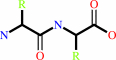
Peptidyl-L-lysine(or L-arginine) + H(2)O = peptide + L-lysine(or L- arginine)
|
 |
 |
 |
 |
 |
|
+
|
|
=
|
|
+
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
44:9339-9347
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of potent thiol-based inhibitors bound to carboxypeptidase B.
|
|
M.Adler,
J.Bryant,
B.Buckman,
I.Islam,
B.Larsen,
S.Finster,
L.Kent,
K.May,
R.Mohan,
S.Yuan,
M.Whitlow.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
This paper presents the crystal structure of porcine pancreatic carboxypeptidase
B (pp-CpB) in complex with a variety of thiol-based inhibitors that were
developed as antagonists of activated thrombin-activatable fibrinolysis
inhibitor (TAFIa). Recent studies have indicated that a selective inhibitor of
TAFIa could enhance the efficacy of existing thrombolytic agents for the
treatment of acute myocardial infarction, one of the most prevalent forms of
heart attacks. Unfortunately, activated TAFIa rapidly degrades in solution and
cannot be used for crystallographic studies. In contrast, porcine pancreatic CpB
is stable at room temperature and is available from commercial sources. Both
pancreatic CpB and TAFIa are zinc-based exopeptidases, and the proteins share a
47% sequence identity. The homology improves considerably in the active site
where nearly all of the residues are conserved. The inhibitors used in this
study were designed to mimic a C-terminal arginine residue, one of the natural
substrates of TAFIa. The X-ray structures show that the thiol group chelates the
active site zinc, the carboxylic acid forms a salt bridge to Arg145, and the
guanidine group forms two hydrogen bonds to Asp255. A meta-substituted phenyl
was introduced into our inhibitors to reduce conformational freedom. This
modification vastly improved the selectivity of compounds against other
exopeptidases that cleave basic residues. Comparisons between structures
indicate that selectivity derives from the interaction between the guanidine
group in the inhibitors and an acidic active site residue. The location of this
acidic residue is not conserved in the various carboxypeptidases.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.M.Grishin,
V.K.h.Akparov,
and
G.G.Chestukhina
(2008).
Leu254 residue and calcium ions as new structural determinants of carboxypeptidase T substrate specificity.
|
| |
Biochemistry (Mosc),
73,
1140-1145.
|
 |
|
|
|
|
 |
M.Adler,
B.Buckman,
J.Bryant,
Z.Chang,
K.Chu,
K.Emayan,
P.Hrvatin,
I.Islam,
J.Morser,
D.Sukovich,
C.West,
S.Yuan,
and
M.Whitlow
(2008).
Structures of potent selective peptide mimetics bound to carboxypeptidase B.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
149-157.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.K.h.Akparov,
A.M.Grishin,
M.P.Yusupova,
N.M.Ivanova,
and
G.G.Chestukhina
(2007).
Structural principles of the wide substrate specificity of Thermoactinomyces vulgaris carboxypeptidase T. Reconstruction of the carboxypeptidase B primary specificity pocket.
|
| |
Biochemistry (Mosc),
72,
416-423.
|
 |
|
|
|
|
 |
P.R.Mittl,
and
M.G.Grütter
(2006).
Opportunities for structure-based design of protease-directed drugs.
|
| |
Curr Opin Struct Biol,
16,
769-775.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

