 |
PDBsum entry 1s3d

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1s3d
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.20.4.1
- arsenate reductase (glutathione/glutaredoxin).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
[glutaredoxin]-dithiol + arsenate + glutathione + H+ = glutathionyl-S- S-[glutaredoxin] + arsenite + H2O
|
 |
 |
 |
 |
 |
[glutaredoxin]-dithiol
|
+
|
 arsenate
arsenate
|
+
|
 glutathione
glutathione
|
+
|
H(+)
|
=
|
glutathionyl-S- S-[glutaredoxin]
|
+
|
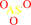 arsenite
arsenite
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mo cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Protein Sci
13:2330-2340
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Arginine 60 in the ArsC arsenate reductase of E. coli plasmid R773 determines the chemical nature of the bound As(III) product.
|
|
S.DeMel,
J.Shi,
P.Martin,
B.P.Rosen,
B.F.Edwards.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Arsenic is a ubiquitous environmental toxic metal. Consequently, organisms
detoxify arsenate by reduction to arsenite, which is then excreted or
sequestered. The ArsC arsenate reductase from Escherichia coli plasmid R773, the
best characterized arsenic-modifying enzyme, has a catalytic cysteine, Cys 12,
in the active site, surrounded by an arginine triad composed of Arg 60, Arg 94,
and Arg 107. During the reaction cycle, the native enzyme forms a unique
monohydroxyl Cys 12-thiol-arsenite adduct that contains a positive charge on the
arsenic. We hypothesized previously that this unstable intermediate allows for
rapid dissociation of the product arsenite. In this study, the role of Arg 60 in
product formation was evaluated by mutagenesis. A total of eight new structures
of ArsC were determined at resolutions between 1.3 A and 1.8 A, with R(free)
values between 0.18 and 0.25. The crystal structures of R60K and R60A ArsC
equilibrated with the product arsenite revealed a covalently bound Cys
12-thiol-dihydroxyarsenite without a charge on the arsenic atom. We propose that
this intermediate is more stable than the monohydroxyarsenite intermediate of
the native enzyme, resulting in slow release of product and, consequently, loss
of activity.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 4.
Figure 4. Interactions of the Csr12 arsonocysteine adducts.
Csr12 and its adjacent residues within 4.0 Å are shown for (A)
native ArsC and (B) the R60K mutant. The hydrogen bonds are
depicted with dotted lines. The cyan atom in Csr12 is arsenic.
|
 |
Figure 5.
Figure 5. Reaction mechanism of the R733 ArsC arsenate
reductase. The mechanism is consistent with the crystal
structures described in Table 1 Go- . In step
1, the free enzyme (structure I) forms the observed covalent
intermediate with arsenate (Martin et al. 2001). In step 2, this
intermediate is glutathionylated, a structure that has not yet
been obtained. In step 3, As(V) is reduced to As(III), producing
a dihydroxy arsenite intermediate (structures VI, IX). In step
4, the novel monohydroxy intermediate with a positively charged
arsenic is formed (Martin et al. 2001). Finally, in step 5, the
free enzyme is regenerated (structure I). . In step
1, the free enzyme (structure I) forms the observed covalent
intermediate with arsenate (Martin et al. 2001). In step 2, this
intermediate is glutathionylated, a structure that has not yet
been obtained. In step 3, As(V) is reduced to As(III), producing
a dihydroxy arsenite intermediate (structures VI, IX). In step
4, the novel monohydroxy intermediate with a positively charged
arsenic is formed (Martin et al. 2001). Finally, in step 5, the
free enzyme is regenerated (structure I).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the Protein Society:
Protein Sci
(2004,
13,
2330-2340)
copyright 2004.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Németi,
and
Z.Gregus
(2009).
Mechanism of thiol-supported arsenate reduction mediated by phosphorolytic-arsenolytic enzymes: I. The role of arsenolysis.
|
| |
Toxicol Sci,
110,
270-281.
|
 |
|
|
|
|
 |
E.Ordóñez,
K.Van Belle,
G.Roos,
S.De Galan,
M.Letek,
J.A.Gil,
L.Wyns,
L.M.Mateos,
and
J.Messens
(2009).
Arsenate reductase, mycothiol, and mycoredoxin concert thiol/disulfide exchange.
|
| |
J Biol Chem,
284,
15107-15116.
|
 |
|
|
|
|
 |
T.T.Ngu,
and
M.J.Stillman
(2009).
Metal-binding mechanisms in metallothioneins.
|
| |
Dalton Trans,
(),
5425-5433.
|
 |
|
|
|
|
 |
G.Roos,
S.Loverix,
E.Brosens,
K.Van Belle,
L.Wyns,
P.Geerlings,
and
J.Messens
(2006).
The activation of electrophile, nucleophile and leaving group during the reaction catalysed by pI258 arsenate reductase.
|
| |
Chembiochem,
7,
981-989.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

