
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, K:
E.C.1.17.1.9
- formate dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
formate + NAD+ = CO2 + NADH
|
 |
 |
 |
 |
 |
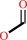 formate
formate
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 75.00% similarity
|
=
|
 CO2
CO2
|
+
|
NADH
Bound ligand (Het Group name = )
matches with 75.00% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Flavin; Iron-sulfur; Mo cation
|
 |
 |
 |
 |
 |
 Flavin
Flavin
|
 Iron-sulfur
Iron-sulfur
|
Mo cation
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
10:1261-1272
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Gene sequence and the 1.8 A crystal structure of the tungsten-containing formate dehydrogenase from Desulfovibrio gigas.
|
|
H.Raaijmakers,
S.Macieira,
J.M.Dias,
S.Teixeira,
S.Bursakov,
R.Huber,
J.J.Moura,
I.Moura,
M.J.Romão.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Desulfovibrio gigas formate dehydrogenase is the first representative of a
tungsten-containing enzyme from a mesophile that has been structurally
characterized. It is a heterodimer of 110 and 24 kDa subunits. The large
subunit, homologous to E. coli FDH-H and to D. desulfuricans nitrate reductase,
center. No small subunit ortholog containing
clusters has been reported. The structural homology with E. coli
FDH-H shows that the essential residues (SeCys158, His159, and Arg407) at the
active site are conserved. The active site is accessible via a positively
charged tunnel, while product release may be facilitated, for H(+) by buried
waters and protonable amino acids and for CO(2) through a hydrophobic channel.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 3.
Figure 3. Stereo Representation of the Overall Structure of
DgW-FDH from D. gigasThe small domain (214 amino acid residues)
is represented in purple, and the large domain (977 amino acid
residues) is colored after its domain classification. Domain I
(red) corresponds to residues 9-68, 575-603, and 647-734; domain
II (green) contains residues 69-157, 410-574, and 604-646;
domain III (yellow) is defined by residues 157-409; domain IV
(blue) is defined by residues 735-977. Domain IV can be divided
into two subdomains, IVa (residues 735-795) and IVb (residues
796-977). The insertions (relative to the homologous NAP and
FDH-H structures) present in several domains are shown in
gray.(B) Representation of the four "exploded" domains of the
large 977 aa subunit.(C) Comparison of the domain organization
of DgW-FDH, D. desulfuricans NAP, and E. coli FDH-H. The
insertions in DgW-FDH are depicted as dashed areas.(D) Stereo Ca
trace numbered every 20 residues for domains II and III
(top).(E) Stereo Ca trace numbered every 20 residues for domains
I and IV and for the small subunit (below).
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from Cell Press:
Structure
(2002,
10,
1261-1272)
copyright 2002.
|
|
| |
Figure was
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
X.Zhang,
E.G.Matson,
and
J.R.Leadbetter
(2011).
Genes for selenium dependent and independent formate dehydrogenase in the gut microbial communities of three lower, wood-feeding termites and a wood-feeding roach.
|
| |
Environ Microbiol,
13,
307-323.
|
 |
|
|
|
|
 |
E.Cremades,
J.Echeverría,
and
S.Alvarez
(2010).
The trigonal prism in coordination chemistry.
|
| |
Chemistry,
16,
10380-10396.
|
 |
|
|
|
|
 |
M.J.Romão
(2009).
Molybdenum and tungsten enzymes: a crystallographic and mechanistic overview.
|
| |
Dalton Trans,
(),
4053-4068.
|
 |
|
|
|
|
 |
N.M.Cerqueira,
P.J.Gonzalez,
C.D.Brondino,
M.J.Romão,
C.C.Romão,
I.Moura,
and
J.J.Moura
(2009).
The effect of the sixth sulfur ligand in the catalytic mechanism of periplasmic nitrate reductase.
|
| |
J Comput Chem,
30,
2466-2484.
|
 |
|
|
|
|
 |
S.Groysman,
and
R.H.Holm
(2009).
Biomimetic Chemistry of Iron, Nickel, Molybdenum, and Tungsten in Sulfur-Ligated Protein Sites (dagger).
|
| |
Biochemistry,
48,
2310-2320.
|
 |
|
|
|
|
 |
H.Sugimoto,
and
H.Tsukube
(2008).
Chemical analogues relevant to molybdenum and tungsten enzyme reaction centres toward structural dynamics and reaction diversity.
|
| |
Chem Soc Rev,
37,
2609-2619.
|
 |
|
|
|
|
 |
J.R.Andreesen,
and
K.Makdessi
(2008).
Tungsten, the surprisingly positively acting heavy metal element for prokaryotes.
|
| |
Ann N Y Acad Sci,
1125,
215-229.
|
 |
|
|
|
|
 |
S.Najmudin,
P.J.González,
J.Trincão,
C.Coelho,
A.Mukhopadhyay,
N.M.Cerqueira,
C.C.Romão,
I.Moura,
J.J.Moura,
C.D.Brondino,
and
M.J.Romão
(2008).
Periplasmic nitrate reductase revisited: a sulfur atom completes the sixth coordination of the catalytic molybdenum.
|
| |
J Biol Inorg Chem,
13,
737-753.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.P.Kloer,
C.Hagel,
J.Heider,
and
G.E.Schulz
(2006).
Crystal structure of ethylbenzene dehydrogenase from Aromatoleum aromaticum.
|
| |
Structure,
14,
1377-1388.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.C.Raaijmakers,
and
M.J.Romão
(2006).
Formate-reduced E. coli formate dehydrogenase H: The reinterpretation of the crystal structure suggests a new reaction mechanism.
|
| |
J Biol Inorg Chem,
11,
849-854.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Boll,
B.Schink,
A.Messerschmidt,
and
P.M.Kroneck
(2005).
Novel bacterial molybdenum and tungsten enzymes: three-dimensional structure, spectroscopy, and reaction mechanism.
|
| |
Biol Chem,
386,
999.
|
 |
|
|
|
|
 |
O.Einsle,
H.Niessen,
D.J.Abt,
G.Seiffert,
B.Schink,
R.Huber,
A.Messerschmidt,
and
P.M.Kroneck
(2005).
Crystallization and preliminary X-ray analysis of the tungsten-dependent acetylene hydratase from Pelobacter acetylenicus.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
61,
299-301.
|
 |
|
|
|
|
 |
F.Peters,
M.Rother,
and
M.Boll
(2004).
Selenocysteine-containing proteins in anaerobic benzoate metabolism of Desulfococcus multivorans.
|
| |
J Bacteriol,
186,
2156-2163.
|
 |
|
|
|
|
 |
J.J.Moura,
C.D.Brondino,
J.Trincão,
and
M.J.Romão
(2004).
Mo and W bis-MGD enzymes: nitrate reductases and formate dehydrogenases.
|
| |
J Biol Inorg Chem,
9,
791-799.
|
 |
|
|
|
|
 |
M.Jormakka,
B.Byrne,
and
S.Iwata
(2003).
Formate dehydrogenase--a versatile enzyme in changing environments.
|
| |
Curr Opin Struct Biol,
13,
418-423.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

