 |
PDBsum entry 6y3c

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
6y3c
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Human cox-1 crystal structure
|
|
Structure:
|
 |
Prostaglandin g/h synthase 1. Chain: a. Synonym: cyclooxygenase-1,cox-1,prostaglandin h2 synthase 1,phs 1, prostaglandin-endoperoxide synthase 1. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: ptgs1, cox1. Expressed in: spodoptera aff. Frugiperda 1 bold-2017. Expression_system_taxid: 2449148
|
|
Resolution:
|
 |
|
3.36Å
|
R-factor:
|
0.214
|
R-free:
|
0.263
|
|
|
Authors:
|
 |
M.Miciaccia,B.D.Belviso,M.Iaselli,S.Ferorelli,M.G.Perrone, R.Caliandro,A.Scilimati
|
|
Key ref:
|
 |
M.Miciaccia
et al.
(2021).
Three-dimensional structure of human cyclooxygenase (hCOX)-1.
Sci Rep,
11,
4312.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
18-Feb-20
|
Release date:
|
26-Feb-20
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P23219
(PGH1_HUMAN) -
Prostaglandin G/H synthase 1 from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
599 a.a.
557 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.99.1
- prostaglandin-endoperoxide synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate + AH2 + 2 O2 = prostaglandin H2 + A + H2O
|
 |
 |
 |
 |
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate
|
+
|
AH2
|
+
|
 2
×
O2
2
×
O2
|
=
|
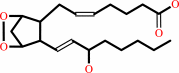 prostaglandin H2
prostaglandin H2
|
+
|
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Sci Rep
11:4312
(2021)
|
|
PubMed id:
|
|
|
|

|
| |
|
Three-dimensional structure of human cyclooxygenase (hCOX)-1.
|
|
M.Miciaccia,
B.D.Belviso,
M.Iaselli,
G.Cingolani,
S.Ferorelli,
M.Cappellari,
P.Loguercio Polosa,
M.G.Perrone,
R.Caliandro,
A.Scilimati.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The beneficial effects of Cyclooxygenases (COX) inhibitors on human health have
been known for thousands of years. Nevertheless, COXs, particularly COX-1, have
been linked to a plethora of human diseases such as cancer, heart failure,
neurological and neurodegenerative diseases only recently. COXs catalyze the
first step in the biosynthesis of prostaglandins (PGs) and are among the most
important mediators of inflammation. All published structural work on COX-1
deals with the ovine isoenzyme, which is easier to produce in
milligram-quantities than the human enzyme and crystallizes readily. Here, we
report the long-sought structure of the human cyclooxygenase-1 (hCOX-1) that we
refined to an R/Rfree of 20.82/26.37, at 3.36 Å resolution. hCOX-1
structure provides a detailed picture of the enzyme active site and the residues
crucial for inhibitor/substrate binding and catalytic activity. We compared
hCOX-1 crystal structure with the ovine COX-1 and human COX-2 structures by
using metrics based on Cartesian coordinates, backbone dihedral angles, and
solvent accessibility coupled with multivariate methods. Differences and
similarities among structures are discussed, with emphasis on the motifs
responsible for the diversification of the various enzymes (primary structure,
stability, catalytic activity, and specificity). The structure of hCOX-1
represents an essential step towards the development of new and more selective
COX-1 inhibitors of enhanced therapeutic potential.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

