 |
PDBsum entry 4m0r

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/transferase inhibitor
|
PDB id
|
|

|

|
4m0r
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.2.18
- anthranilate phosphoribosyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-(5-phospho-beta-D-ribosyl)anthranilate + diphosphate = 5-phospho-alpha- D-ribose 1-diphosphate + anthranilate
|
 |
 |
 |
 |
 |
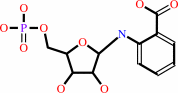 N-(5-phospho-beta-D-ribosyl)anthranilate
N-(5-phospho-beta-D-ribosyl)anthranilate
|
+
|
diphosphate
Bound ligand (Het Group name = )
matches with 40.54% similarity
|
=
|
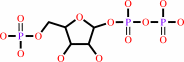 5-phospho-alpha- D-ribose 1-diphosphate
5-phospho-alpha- D-ribose 1-diphosphate
|
+
|
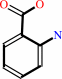 anthranilate
anthranilate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Chembiochem
15:852-864
(2014)
|
|
PubMed id:
|
|
|
|

|
| |
|
Repurposing the chemical scaffold of the anti-arthritic drug Lobenzarit to target tryptophan biosynthesis in Mycobacterium tuberculosis.
|
|
G.L.Evans,
S.A.Gamage,
E.M.Bulloch,
E.N.Baker,
W.A.Denny,
J.S.Lott.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The emergence of extensively drug-resistant strains of Mycobacterium
tuberculosis (Mtb) highlights the need for new therapeutics to treat
tuberculosis. We are attempting to fast-track a targeted approach to drug design
by generating analogues of a validated hit from molecular library screening that
shares its chemical scaffold with a current therapeutic, the anti-arthritic drug
Lobenzarit (LBZ). Our target, anthranilate phosphoribosyltransferase (AnPRT), is
an enzyme from the tryptophan biosynthetic pathway in Mtb. A bifurcated hydrogen
bond was found to be a key feature of the LBZ-like chemical scaffold and
critical for enzyme inhibition. We have determined crystal structures of
compounds in complex with the enzyme that indicate that the bifurcated hydrogen
bond assists in orientating compounds in the correct conformation to interact
with key residues in the substrate-binding tunnel of Mtb-AnPRT. Characterising
the inhibitory potency of the hit and its analogues in different ways proved
useful, due to the multiple substrates and substrate binding sites of this
enzyme. Binding in a site other than the catalytic site was found to be
associated with partial inhibition. An analogue,
2-(2-5-methylcarboxyphenylamino)-3-methylbenzoic acid, that bound at the
catalytic site and caused complete, rather than partial, inhibition of enzyme
activity was found. Therefore, we designed and synthesised an extended version
of the scaffold on the basis of this observation. The resultant compound,
2,6-bis-(2-carboxyphenylamino)benzoate, is a 40-fold more potent inhibitor of
the enzyme than the original hit and provides direction for further
structure-based drug design.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

