 |
PDBsum entry 2rdw

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2rdw
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.1.1.3.15
- (S)-2-hydroxy-acid oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a (2S)-2-hydroxycarboxylate + O2 = a 2-oxocarboxylate + H2O2
|
 |
 |
 |
 |
 |
(2S)-2-hydroxycarboxylate
|
+
|
 O2
O2
|
=
|
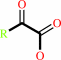 2-oxocarboxylate
2-oxocarboxylate
|
+
|
 H2O2
H2O2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FMN
|
 |
 |
 |
 |
 |
FMN
Bound ligand (Het Group name =
FMN)
corresponds exactly
|
|
 |
 |
Enzyme class 2:
|
 |
E.C.1.2.3.5
- glyoxylate oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
glyoxylate + O2 + H2O = oxalate + H2O2 + H+
|
 |
 |
 |
 |
 |
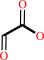 glyoxylate
glyoxylate
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
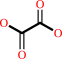 oxalate
oxalate
|
+
|
 H2O2
H2O2
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
47:2439-2449
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Active site and loop 4 movements within human glycolate oxidase: implications for substrate specificity and drug design.
|
|
M.S.Murray,
R.P.Holmes,
W.T.Lowther.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Human glycolate oxidase (GO) catalyzes the FMN-dependent oxidation of glycolate
to glyoxylate and glyoxylate to oxalate, a key metabolite in kidney stone
formation. We report herein the structures of recombinant GO complexed with
sulfate, glyoxylate, and an inhibitor,
4-carboxy-5-dodecylsulfanyl-1,2,3-triazole (CDST), determined by X-ray
crystallography. In contrast to most alpha-hydroxy acid oxidases including
spinach glycolate oxidase, a loop region, known as loop 4, is completely visible
when the GO active site contains a small ligand. The lack of electron density
for this loop in the GO-CDST complex, which mimics a large substrate, suggests
that a disordered to ordered transition may occur with the binding of
substrates. The conformational flexibility of Trp110 appears to be responsible
for enabling GO to react with alpha-hydroxy acids of various chain lengths.
Moreover, the movement of Trp110 disrupts a hydrogen-bonding network between
Trp110, Leu191, Tyr134, and Tyr208. This loss of interactions is the first
indication that active site movements are directly linked to changes in the
conformation of loop 4. The kinetic parameters for the oxidation of glycolate,
glyoxylate, and 2-hydroxy octanoate indicate that the oxidation of glycolate to
glyoxylate is the primary reaction catalyzed by GO, while the oxidation of
glyoxylate to oxalate is most likely not relevant under normal conditions.
However, drugs that exploit the unique structural features of GO may ultimately
prove to be useful for decreasing glycolate and glyoxylate levels in primary
hyperoxaluria type 1 patients who have the inability to convert peroxisomal
glyoxylate to glycine.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Pennati,
and
G.Gadda
(2009).
Involvement of ionizable groups in catalysis of human liver glycolate oxidase.
|
| |
J Biol Chem,
284,
31214-31222.
|
 |
|
|
|
|
 |
S.Donini,
M.Ferrari,
C.Fedeli,
M.Faini,
I.Lamberto,
A.S.Marletta,
L.Mellini,
M.Panini,
R.Percudani,
L.Pollegioni,
L.Caldinelli,
S.Petrucco,
and
A.Peracchi
(2009).
Recombinant production of eight human cytosolic aminotransferases and assessment of their potential involvement in glyoxylate metabolism.
|
| |
Biochem J,
422,
265-272.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

