 |
PDBsum entry 2oa6

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.3.9
- aristolochene synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Germacrene derived sesquiterpenoid biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(2E,6E)-farnesyl diphosphate = +-aristolochene + diphosphate
|
 |
 |
 |
 |
 |
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
=
|
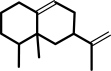 (+)-aristolochene
(+)-aristolochene
|
+
|
diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+); Mn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
46:1941-1951
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
X-ray crystal structure of aristolochene synthase from Aspergillus terreus and evolution of templates for the cyclization of farnesyl diphosphate.
|
|
E.Y.Shishova,
L.Di Costanzo,
D.E.Cane,
D.W.Christianson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Aristolochene synthase from Aspergillus terreus catalyzes the cyclization of the
universal sesquiterpene precursor, farnesyl diphosphate, to form the bicyclic
hydrocarbon aristolochene. The 2.2 A resolution X-ray crystal structure of
aristolochene synthase reveals a tetrameric quaternary structure in which each
subunit adopts the alpha-helical class I terpene synthase fold with the active
site in the "open", solvent-exposed conformation. Intriguingly, the 2.15 A
resolution crystal structure of the complex with Mg2+3-pyrophosphate reveals
ligand binding only to tetramer subunit D, which is stabilized in the "closed"
conformation required for catalysis. Tetramer assembly may hinder conformational
changes required for the transition from the inactive open conformation to the
active closed conformation, thereby accounting for the attenuation of catalytic
activity with an increase in enzyme concentration. In both conformations, but
especially in the closed conformation, the active site contour is highly
complementary in shape to that of aristolochene, and a catalytic function is
proposed for the pyrophosphate anion based on its orientation with regard to the
presumed binding mode of aristolochene. A similar active site contour is
conserved in aristolochene synthase from Penicillium roqueforti despite the
substantial divergent evolution of these two enzymes, while strikingly different
active site contours are found in the sesquiterpene cyclases 5-epi-aristolochene
synthase and trichodiene synthase. Thus, the terpenoid cyclase active site plays
a critical role as a template in binding the flexible polyisoprenoid substrate
in the proper conformation for catalysis. Across the greater family of terpenoid
cyclases, this template is highly evolvable within a conserved alpha-helical
fold for the synthesis of terpene natural products of diverse structure and
stereochemistry.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Engels,
U.Heinig,
T.Grothe,
M.Stadler,
and
S.Jennewein
(2011).
Cloning and Characterization of an Armillaria gallica cDNA Encoding Protoilludene Synthase, Which Catalyzes the First Committed Step in the Synthesis of Antimicrobial Melleolides.
|
| |
J Biol Chem,
286,
6871-6878.
|
 |
|
|
|
|
 |
K.Zhou,
and
R.J.Peters
(2011).
Electrostatic effects on (di)terpene synthase product outcome.
|
| |
Chem Commun (Camb),
47,
4074-4080.
|
 |
|
|
|
|
 |
F.Lopez-Gallego,
S.A.Agger,
D.Abate-Pella,
M.D.Distefano,
and
C.Schmidt-Dannert
(2010).
Sesquiterpene synthases Cop4 and Cop6 from Coprinus cinereus: catalytic promiscuity and cyclization of farnesyl pyrophosphate geometric isomers.
|
| |
Chembiochem,
11,
1093-1106.
|
 |
|
|
|
|
 |
J.A.Aaron,
X.Lin,
D.E.Cane,
and
D.W.Christianson
(2010).
Structure of epi-isozizaene synthase from Streptomyces coelicolor A3(2), a platform for new terpenoid cyclization templates.
|
| |
Biochemistry,
49,
1787-1797.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.P.Noel,
N.Dellas,
J.A.Faraldos,
M.Zhao,
B.A.Hess,
L.Smentek,
R.M.Coates,
and
P.E.O'Maille
(2010).
Structural elucidation of cisoid and transoid cyclization pathways of a sesquiterpene synthase using 2-fluorofarnesyl diphosphates.
|
| |
ACS Chem Biol,
5,
377-392.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.J.Hong,
and
D.J.Tantillo
(2010).
Quantum chemical dissection of the classic terpinyl/pinyl/bornyl/camphyl cation conundrum-the role of pyrophosphate in manipulating pathways to monoterpenes.
|
| |
Org Biomol Chem,
8,
4589-4600.
|
 |
|
|
|
|
 |
B.Zhao,
L.Lei,
D.G.Vassylyev,
X.Lin,
D.E.Cane,
S.L.Kelly,
H.Yuan,
D.C.Lamb,
and
M.R.Waterman
(2009).
Crystal structure of albaflavenone monooxygenase containing a moonlighting terpene synthase active site.
|
| |
J Biol Chem,
284,
36711-36719.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.A.Gennadios,
V.Gonzalez,
L.Di Costanzo,
A.Li,
F.Yu,
D.J.Miller,
R.K.Allemann,
and
D.W.Christianson
(2009).
Crystal structure of (+)-delta-cadinene synthase from Gossypium arboreum and evolutionary divergence of metal binding motifs for catalysis.
|
| |
Biochemistry,
48,
6175-6183.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Agger,
F.Lopez-Gallego,
and
C.Schmidt-Dannert
(2009).
Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus.
|
| |
Mol Microbiol,
72,
1181-1195.
|
 |
|
|
|
|
 |
S.Green,
C.J.Squire,
N.J.Nieuwenhuizen,
E.N.Baker,
and
W.Laing
(2009).
Defining the potassium binding region in an apple terpene synthase.
|
| |
J Biol Chem,
284,
8661-8669.
|
 |
|
|
|
|
 |
S.Y.Kim,
P.Zhao,
M.Igarashi,
R.Sawa,
T.Tomita,
M.Nishiyama,
and
T.Kuzuyama
(2009).
Cloning and heterologous expression of the cyclooctatin biosynthetic gene cluster afford a diterpene cyclase and two p450 hydroxylases.
|
| |
Chem Biol,
16,
736-743.
|
 |
|
|
|
|
 |
B.M.Fraga
(2008).
Natural sesquiterpenoids.
|
| |
Nat Prod Rep,
25,
1180-1209.
|
 |
|
|
|
|
 |
D.J.Miller,
J.Gao,
D.G.Truhlar,
N.J.Young,
V.Gonzalez,
and
R.K.Allemann
(2008).
Stereochemistry of eudesmane cation formation during catalysis by aristolochene synthase from Penicillium roqueforti.
|
| |
Org Biomol Chem,
6,
2346-2354.
|
 |
|
|
|
|
 |
D.Lubertozzi,
and
J.D.Keasling
(2008).
Expression of a synthetic Artemesia annua amorphadiene synthase in Aspergillus nidulans yields altered product distribution.
|
| |
J Ind Microbiol Biotechnol,
35,
1191-1198.
|
 |
|
|
|
|
 |
D.W.Christianson
(2008).
Unearthing the roots of the terpenome.
|
| |
Curr Opin Chem Biol,
12,
141-150.
|
 |
|
|
|
|
 |
E.Y.Shishova,
F.Yu,
D.J.Miller,
J.A.Faraldos,
Y.Zhao,
R.M.Coates,
R.K.Allemann,
D.E.Cane,
and
D.W.Christianson
(2008).
X-ray crystallographic studies of substrate binding to aristolochene synthase suggest a metal ion binding sequence for catalysis.
|
| |
J Biol Chem,
283,
15431-15439.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.L.Lairson,
B.Henrissat,
G.J.Davies,
and
S.G.Withers
(2008).
Glycosyltransferases: structures, functions, and mechanisms.
|
| |
Annu Rev Biochem,
77,
521-555.
|
 |
|
|
|
|
 |
L.S.Vedula,
J.Jiang,
T.Zakharian,
D.E.Cane,
and
D.W.Christianson
(2008).
Structural and mechanistic analysis of trichodiene synthase using site-directed mutagenesis: probing the catalytic function of tyrosine-295 and the asparagine-225/serine-229/glutamate-233-Mg2+B motif.
|
| |
Arch Biochem Biophys,
469,
184-194.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.A.Agger,
F.Lopez-Gallego,
T.R.Hoye,
and
C.Schmidt-Dannert
(2008).
Identification of sesquiterpene synthases from Nostoc punctiforme PCC 73102 and Nostoc sp. strain PCC 7120.
|
| |
J Bacteriol,
190,
6084-6096.
|
 |
|
|
|
|
 |
T.G.Köllner,
C.Schnee,
S.Li,
A.Svatos,
B.Schneider,
J.Gershenzon,
and
J.Degenhardt
(2008).
Protonation of a neutral (S)-beta-bisabolene intermediate is involved in (S)-beta-macrocarpene formation by the maize sesquiterpene synthases TPS6 and TPS11.
|
| |
J Biol Chem,
283,
20779-20788.
|
 |
|
|
|
|
 |
D.J.Miller,
F.Yu,
and
R.K.Allemann
(2007).
Aristolochene synthase-catalyzed cyclization of 2-fluorofarnesyl-diphosphate to 2-fluorogermacrene A.
|
| |
Chembiochem,
8,
1819-1825.
|
 |
|
|
|
|
 |
F.Yu,
D.J.Miller,
and
R.K.Allemann
(2007).
Probing the reaction mechanism of aristolochene synthase with 12,13-difluorofarnesyl diphosphate.
|
| |
Chem Commun (Camb),
(),
4155-4157.
|
 |
|
|
|
|
 |
R.K.Allemann,
N.J.Young,
S.Ma,
D.G.Truhlar,
and
J.Gao
(2007).
Synthetic efficiency in enzyme mechanisms involving carbocations: aristolochene synthase.
|
| |
J Am Chem Soc,
129,
13008-13013.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

