 |
PDBsum entry 1qi9

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1qi9
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.18
- bromide peroxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RH + Br- + H2O2 = RBr + 2 H2O
|
 |
 |
 |
 |
 |
RH
|
+
|
Br(-)
|
+
|
 H2O2
H2O2
|
=
|
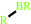 RBr
RBr
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
293:595-611
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
X-ray structure determination of a vanadium-dependent haloperoxidase from Ascophyllum nodosum at 2.0 A resolution.
|
|
M.Weyand,
H.Hecht,
M.Kiess,
M.Liaud,
H.Vilter,
D.Schomburg.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The homo-dimeric structure of a vanadium-dependent haloperoxidase (V-BPO) from
the brown alga Ascophyllum nodosum (EC 1.1.11.X) has been solved by single
isomorphous replacement anomalous scattering (SIRAS) X-ray crystallography at
2.0 A resolution (PDB accession code 1QI9), using two heavy-atom datasets of a
tungstate derivative measured at two different wavelengths. The protein sequence
(SwissProt entry code P81701) of V-BPO was established by combining results from
protein and DNA sequencing, and electron density interpretation. The enzyme has
nearly an all-helical structure, with two four-helix bundles and only three
small beta-sheets. The holoenzyme contains trigonal-bipyramidal coordinated
vanadium atoms at its two active centres. Structural similarity to the only
other structurally characterized vanadium-dependent chloroperoxidase (V-CPO)
from Curvularia inaequalis exists in the vicinity of the active site and to a
lesser extent in the central four-helix bundle. Despite the low sequence and
structural similarity between V-BPO and V-CPO, the vanadium binding centres are
highly conserved on the N-terminal side of an alpha-helix and include the
proposed catalytic histidine residue (His418(V-BPO)/His404(V-CPO)). The V-BPO
structure contains, in addition, a second histidine near the active site
(His411(V-BPO)), which can alter the redox potential of the catalytically active
VO2-O2 species by protonation/deprotonation reactions. Specific binding sites
for the organic substrates, like indoles and monochlordimedone, or for halide
ions are not visible in the V-BPO structure. A reaction mechanism for the
enzymatic oxidation of halides is discussed, based on the present structural,
spectroscopic and biochemical knowledge of vanadium-dependent haloperoxidases,
explaining the observed enzymatic differences between both enzymes.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 8.
Figure 8. Structure alignment of V-CPO and V-BPO. Residues with special functions are in coloured bold letters:
green, connected to vanadium atom; red, hydrogen-bonded to vanadate oxygen atoms; catalytic active histidine resi-
dues, pink (conserved in V-HPO) and blue (unique in V-BPO). C
a
-pairs used in matrix and RMS deviation calcu-
lations are marked by asterisks (*). The tertiary structure alignment (Lessel & Schomburg, 1994) and the Figure was
prepared using BRAGI (Schomburg & Reichelt, 1988).
|
 |
Figure 10.
Figure 10. Proposal of a common
reaction mechanism for the halide
oxidation by vanadium-dependent
haloperoxidases. See Discussion.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(1999,
293,
595-611)
copyright 1999.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.G.Werncke,
C.Limberg,
C.Knispel,
R.Metzinger,
and
B.Braun
(2011).
Haloperoxidase activity of oxovanadium(v) thiobisphenolates.
|
| |
Chemistry,
17,
2931-2938.
|
 |
|
|
|
|
 |
M.R.Maurya,
A.A.Khan,
A.Azam,
S.Ranjan,
N.Mondal,
A.Kumar,
F.Avecilla,
and
J.C.Pessoa
(2010).
Vanadium complexes having [V(IV)O](2+) and [V(V)O(2)](+) cores with binucleating dibasic tetradentate ligands: Synthesis, characterization, catalytic and antiamoebic activities.
|
| |
Dalton Trans,
39,
1345-1360.
|
 |
|
|
|
|
 |
A.Butler,
and
M.Sandy
(2009).
Mechanistic considerations of halogenating enzymes.
|
| |
Nature,
460,
848-854.
|
 |
|
|
|
|
 |
J.M.Winter,
and
B.S.Moore
(2009).
Exploring the Chemistry and Biology of Vanadium-dependent Haloperoxidases.
|
| |
J Biol Chem,
284,
18577-18581.
|
 |
|
|
|
|
 |
M.Bernroitner,
M.Zamocky,
P.G.Furtmüller,
G.A.Peschek,
and
C.Obinger
(2009).
Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria.
|
| |
J Exp Bot,
60,
423-440.
|
 |
|
|
|
|
 |
M.R.Maurya,
A.Arya,
A.Kumar,
and
J.C.Pessoa
(2009).
Polystyrene bound oxidovanadium(IV) and dioxidovanadium(V) complexes of histamine derived ligand for the oxidation of methyl phenyl sulfide, diphenyl sulfide and benzoin.
|
| |
Dalton Trans,
(),
2185-2195.
|
 |
|
|
|
|
 |
Y.Chen,
J.Jakoncic,
K.A.Parker,
N.Carpino,
and
N.Nassar
(2009).
Structures of the phosphorylated and VO(3)-bound 2H-phosphatase domain of Sts-2.
|
| |
Biochemistry,
48,
8129-8135.
|
 |
|
|
|
|
 |
C.S.Neumann,
D.G.Fujimori,
and
C.T.Walsh
(2008).
Halogenation strategies in natural product biosynthesis.
|
| |
Chem Biol,
15,
99.
|
 |
|
|
|
|
 |
D.Rehder
(2008).
Is vanadium a more versatile target in the activity of primordial life forms than hitherto anticipated?
|
| |
Org Biomol Chem,
6,
957-964.
|
 |
|
|
|
|
 |
P.Wu,
G.Santoni,
M.Fröba,
and
D.Rehder
(2008).
Modelling the sulfoxygenation activity of vanadate-dependent peroxidases.
|
| |
Chem Biodivers,
5,
1913-1926.
|
 |
|
|
|
|
 |
B.S.Moore
(2006).
Biosynthesis of marine natural products: macroorganisms (Part B).
|
| |
Nat Prod Rep,
23,
615-629.
|
 |
|
|
|
|
 |
J.M.Notestein,
and
A.Katz
(2006).
Enhancing heterogeneous catalysis through cooperative hybrid organic-inorganic interfaces.
|
| |
Chemistry,
12,
3954-3965.
|
 |
|
|
|
|
 |
M.R.Maurya,
S.Agarwal,
M.Abid,
A.Azam,
C.Bader,
M.Ebel,
and
D.Rehder
(2006).
Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes.
|
| |
Dalton Trans,
(),
937-947.
|
 |
|
|
|
|
 |
M.R.Maurya,
U.Kumar,
and
P.Manikandan
(2006).
Polymer supported vanadium and molybdenum complexes as potential catalysts for the oxidation and oxidative bromination of organic substrates.
|
| |
Dalton Trans,
(),
3561-3575.
|
 |
|
|
|
|
 |
C.Colin,
C.Leblanc,
G.Michel,
E.Wagner,
E.Leize-Wagner,
A.Van Dorsselaer,
and
P.Potin
(2005).
Vanadium-dependent iodoperoxidases in Laminaria digitata, a novel biochemical function diverging from brown algal bromoperoxidases.
|
| |
J Biol Inorg Chem,
10,
156-166.
|
 |
|
|
|
|
 |
E.Garcia-Rodriguez,
T.Ohshiro,
T.Aibara,
Y.Izumi,
and
J.Littlechild
(2005).
Enhancing effect of calcium and vanadium ions on thermal stability of bromoperoxidase from Corallina pilulifera.
|
| |
J Biol Inorg Chem,
10,
275-282.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.R.Maurya,
S.Agarwal,
C.Bader,
M.Ebel,
and
D.Rehder
(2005).
Synthesis, characterisation and catalytic potential of hydrazonato-vanadium(V) model complexes with [VO]3+ and [VO2]+ cores.
|
| |
Dalton Trans,
(),
537-544.
|
 |
|
|
|
|
 |
T.Ohshiro,
J.Littlechild,
E.Garcia-Rodriguez,
M.N.Isupov,
Y.Iida,
T.Kobayashi,
and
Y.Izumi
(2004).
Modification of halogen specificity of a vanadium-dependent bromoperoxidase.
|
| |
Protein Sci,
13,
1566-1571.
|
 |
|
|
|
|
 |
C.Colin,
C.Leblanc,
E.Wagner,
L.Delage,
E.Leize-Wagner,
A.Van Dorsselaer,
B.Kloareg,
and
P.Potin
(2003).
The brown algal kelp Laminaria digitata features distinct bromoperoxidase and iodoperoxidase activities.
|
| |
J Biol Chem,
278,
23545-23552.
|
 |
|
|
|
|
 |
C.Naumann,
T.Hartmann,
and
D.Ober
(2002).
Evolutionary recruitment of a flavin-dependent monooxygenase for the detoxification of host plant-acquired pyrrolizidine alkaloids in the alkaloid-defended arctiid moth Tyria jacobaeae.
|
| |
Proc Natl Acad Sci U S A,
99,
6085-6090.
|
 |
|
|
|
|
 |
J.Littlechild,
E.Garcia-Rodriguez,
A.Dalby,
and
M.Isupov
(2002).
Structural and functional comparisons between vanadium haloperoxidase and acid phosphatase enzymes.
|
| |
J Mol Recognit,
15,
291-296.
|
 |
|
|
|
|
 |
M.Piepenbrink,
M.U.Triller,
N.H.Gorman,
and
B.Krebs
(2002).
Bridging the gap between polyoxometalates and classic coordination compounds: a novel type of hexavanadate complex.
|
| |
Angew Chem Int Ed Engl,
41,
2523-2525.
|
 |
|
|
|
|
 |
N.Bar-Nun,
S.Shcolnick,
and
A.M.Mayer
(2002).
Presence of a vanadium-dependent haloperoxidase in Botrytis cinerea.
|
| |
FEMS Microbiol Lett,
217,
121-124.
|
 |
|
|
|
|
 |
N.Tanaka,
V.Dumay,
Q.Liao,
A.J.Lange,
and
R.Wever
(2002).
Bromoperoxidase activity of vanadate-substituted acid phosphatases from Shigella flexneri and Salmonella enterica ser. typhimurium.
|
| |
Eur J Biochem,
269,
2162-2167.
|
 |
|
|
|
|
 |
P.Potin,
K.Bouarab,
J.P.Salaün,
G.Pohnert,
and
B.Kloareg
(2002).
Biotic interactions of marine algae.
|
| |
Curr Opin Plant Biol,
5,
308-317.
|
 |
|
|
|
|
 |
H.B.ten Brink,
H.E.Schoemaker,
and
R.Wever
(2001).
Sulfoxidation mechanism of vanadium bromoperoxidase from Ascophyllum nodosum. Evidence for direct oxygen transfer catalysis.
|
| |
Eur J Biochem,
268,
132-138.
|
 |
|
|
|
|
 |
R.Renirie,
W.Hemrika,
and
R.Wever
(2000).
Peroxidase and phosphatase activity of active-site mutants of vanadium chloroperoxidase from the fungus Curvularia inaequalis. Implications for the catalytic mechanisms.
|
| |
J Biol Chem,
275,
11650-11657.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

