
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chain A:
E.C.1.14.13.39
- nitric-oxide synthase (NADPH).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 L-arginine + 3 NADPH + 4 O2 + H+ = 2 L-citrulline + 2 nitric oxide + 3 NADP+ + 4 H2O
|
 |
 |
 |
 |
 |
 2
×
L-arginine
2
×
L-arginine
|
+
|
3
×
NADPH
Bound ligand (Het Group name = )
matches with 41.67% similarity
|
+
|
 4
×
O2
4
×
O2
|
+
|
H(+)
|
=
|
 2
×
L-citrulline
2
×
L-citrulline
|
+
|
 2
×
nitric oxide
2
×
nitric oxide
|
+
|
 3
×
NADP(+)
3
×
NADP(+)
|
+
|
4
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chain B:
E.C.2.3.1.28
- chloramphenicol O-acetyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
chloramphenicol + acetyl-CoA = chloramphenicol 3-acetate + CoA
|
 |
 |
 |
 |
 |
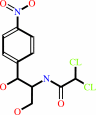 2
×
chloramphenicol
2
×
chloramphenicol
|
+
|
 3
×
acetyl-CoA
3
×
acetyl-CoA
|
=
|
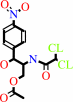 4
×
chloramphenicol 3-acetate
4
×
chloramphenicol 3-acetate
|
+
|
 CoA
CoA
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Science
278:425-431
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of nitric oxide synthase oxygenase domain and inhibitor complexes.
|
|
B.R.Crane,
A.S.Arvai,
R.Gachhui,
C.Wu,
D.K.Ghosh,
E.D.Getzoff,
D.J.Stuehr,
J.A.Tainer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The nitric oxide synthase oxygenase domain (NOSox) oxidizes arginine to
synthesize the cellular signal and defensive cytotoxin nitric oxide (NO).
Crystal structures determined for cytokine-inducible NOSox reveal an unusual
fold and heme environment for stabilization of activated oxygen intermediates
key for catalysis. A winged beta sheet engenders a curved alpha-beta domain
resembling a baseball catcher's mitt with heme clasped in the palm. The location
of exposed hydrophobic residues and the results of mutational analysis place the
dimer interface adjacent to the heme-binding pocket. Juxtaposed hydrophobic O2-
and polar L-arginine-binding sites occupied by imidazole and aminoguanidine,
respectively, provide a template for designing dual-function inhibitors and
imply substrate-assisted catalysis.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Fig. 3. Mobility, surface properties, and shape. (A) C  trace of
NOS[ox] trace of
NOS[ox]  114 (cubic
crystal form) colored by the crystallographic^ temperature
factor (low to high B factors colored blue to red) and displayed
with heme and mutation sites that affect function. Mutation
sites (side chains displayed and labeled by residue number)
affecting dimerization, L-Arg binding, or H[4]B binding
(defined^ in Fig. 2) cluster to highly mobile (red) projecting
regions. The view is rotated by about 45° from Fig. 1 about
a vertical axis. (B) Solvent-accessible molecular surface of
flattened^ (left) and concave (center) face. The orientation is
the same^ as in (A). The exposed heme edge (gold), residues
contributing to the distal pocket (cyan), and exposed conserved
hydrophobic^ residues (green) (defined in Fig. 2) map to the
same flattened^ face of the molecule and cluster in the regions
of high mobility and mutational sensitivity shown in (A), making
this surface the^ prime candidate for a symmetric dimer
interface. (C) Solvent-accessible^ molecular surface of the
narrow curved face. This face has few conserved exposed
hydrophobic residues. The view is rotated 90° from (A) and
(B) around a vertical axis. 114 (cubic
crystal form) colored by the crystallographic^ temperature
factor (low to high B factors colored blue to red) and displayed
with heme and mutation sites that affect function. Mutation
sites (side chains displayed and labeled by residue number)
affecting dimerization, L-Arg binding, or H[4]B binding
(defined^ in Fig. 2) cluster to highly mobile (red) projecting
regions. The view is rotated by about 45° from Fig. 1 about
a vertical axis. (B) Solvent-accessible molecular surface of
flattened^ (left) and concave (center) face. The orientation is
the same^ as in (A). The exposed heme edge (gold), residues
contributing to the distal pocket (cyan), and exposed conserved
hydrophobic^ residues (green) (defined in Fig. 2) map to the
same flattened^ face of the molecule and cluster in the regions
of high mobility and mutational sensitivity shown in (A), making
this surface the^ prime candidate for a symmetric dimer
interface. (C) Solvent-accessible^ molecular surface of the
narrow curved face. This face has few conserved exposed
hydrophobic residues. The view is rotated 90° from (A) and
(B) around a vertical axis.
|
 |
Figure 5.
Fig. 5. Comparison of the proximal heme-binding regions of
iNOS[ox] and cytochrome P450s. Structural elements contributing
to the proximal heme-binding regions of iNOS[ox]  114 and
P450[cam] (cyan C 114 and
P450[cam] (cyan C  traces)
are substantially different. Only the proximal Cys ligands
(magenta bonds with yellow sulfur atoms, bound to gold^ hemes)
and immediately COOH-terminal three residues (magenta C traces)
are substantially different. Only the proximal Cys ligands
(magenta bonds with yellow sulfur atoms, bound to gold^ hemes)
and immediately COOH-terminal three residues (magenta C  traces)
have similar conformations. In iNOS[ox], Cys194 lies at the
COOH-terminal end of a helix and precedes an extended^ strand,
whereas in P450[cam], Cys357 lies at the NH[2]-terminal end of a
helix and follows an extended^ strand. Also, these two cysteine
thiolates bind opposite faces of iron protoporphyrin IX. C traces)
have similar conformations. In iNOS[ox], Cys194 lies at the
COOH-terminal end of a helix and precedes an extended^ strand,
whereas in P450[cam], Cys357 lies at the NH[2]-terminal end of a
helix and follows an extended^ strand. Also, these two cysteine
thiolates bind opposite faces of iron protoporphyrin IX. C  positions
for iNOS[ox] positions
for iNOS[ox]  114
residues 194 to 197 were superimposed with P450[cam] residues
357^ to 360 and then separated for clarity. 114
residues 194 to 197 were superimposed with P450[cam] residues
357^ to 360 and then separated for clarity.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the AAAs:
Science
(1997,
278,
425-431)
copyright 1997.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.R.Crane,
J.Sudhamsu,
and
B.A.Patel
(2010).
Bacterial nitric oxide synthases.
|
| |
Annu Rev Biochem,
79,
445-470.
|
 |
|
|
|
|
 |
D.Schade,
J.Kotthaus,
and
B.Clement
(2010).
Modulating the NO generating system from a medicinal chemistry perspective: current trends and therapeutic options in cardiovascular disease.
|
| |
Pharmacol Ther,
126,
279-300.
|
 |
|
|
|
|
 |
D.J.Stuehr,
J.Tejero,
and
M.M.Haque
(2009).
Structural and mechanistic aspects of flavoproteins: electron transfer through the nitric oxide synthase flavoprotein domain.
|
| |
FEBS J,
276,
3959-3974.
|
 |
|
|
|
|
 |
M.M.Haque,
M.Fadlalla,
Z.Q.Wang,
S.S.Ray,
K.Panda,
and
D.J.Stuehr
(2009).
Neutralizing a surface charge on the FMN subdomain increases the activity of neuronal nitric-oxide synthase by enhancing the oxygen reactivity of the enzyme heme-nitric oxide complex.
|
| |
J Biol Chem,
284,
19237-19247.
|
 |
|
|
|
|
 |
R.B.Silverman
(2009).
Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases.
|
| |
Acc Chem Res,
42,
439-451.
|
 |
|
|
|
|
 |
B.Ozüyaman,
M.Grau,
M.Kelm,
M.W.Merx,
and
P.Kleinbongard
(2008).
RBC NOS: regulatory mechanisms and therapeutic aspects.
|
| |
Trends Mol Med,
14,
314-322.
|
 |
|
|
|
|
 |
E.D.Garcin,
A.S.Arvai,
R.J.Rosenfeld,
M.D.Kroeger,
B.R.Crane,
G.Andersson,
G.Andrews,
P.J.Hamley,
P.R.Mallinder,
D.J.Nicholls,
S.A.St-Gallay,
A.C.Tinker,
N.P.Gensmantel,
A.Mete,
D.R.Cheshire,
S.Connolly,
D.J.Stuehr,
A.Aberg,
A.V.Wallace,
J.A.Tainer,
and
E.D.Getzoff
(2008).
Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase.
|
| |
Nat Chem Biol,
4,
700-707.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Ohtsuki,
J.Yokoyama,
N.Ohba,
Y.Ohmiya,
and
M.Kawata
(2008).
Nitric oxide synthase (NOS) in the Japanese fireflies Luciola lateralis and Luciola cruciata.
|
| |
Arch Insect Biochem Physiol,
69,
176-188.
|
 |
|
|
|
|
 |
J.Tejero,
A.Biswas,
Z.Q.Wang,
R.C.Page,
M.M.Haque,
C.Hemann,
J.L.Zweier,
S.Misra,
and
D.J.Stuehr
(2008).
Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase.
|
| |
J Biol Chem,
283,
33498-33507.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.M.Francis,
A.Mittal,
M.Sharma,
and
P.V.Bharatam
(2008).
Design of benzene-1,2-diamines as selective inducible nitric oxide synthase inhibitors: a combined de novo design and docking analysis.
|
| |
J Mol Model,
14,
215-224.
|
 |
|
|
|
|
 |
A.W.Munro,
H.M.Girvan,
and
K.J.McLean
(2007).
Variations on a (t)heme--novel mechanisms, redox partners and catalytic functions in the cytochrome P450 superfamily.
|
| |
Nat Prod Rep,
24,
585-609.
|
 |
|
|
|
|
 |
C.Wheatley
(2007).
The return of the Scarlet Pimpernel: cobalamin in inflammation II - cobalamins can both selectively promote all three nitric oxide synthases (NOS), particularly iNOS and eNOS, and, as needed, selectively inhibit iNOS and nNOS.
|
| |
J Nutr Environ Med,
16,
181-211.
|
 |
|
|
|
|
 |
C.Wheatley
(2007).
Cobalamin in inflammation III - glutathionylcobalamin and methylcobalamin/adenosylcobalamin coenzymes: the sword in the stone? How cobalamin may directly regulate the nitric oxide synthases.
|
| |
J Nutr Environ Med,
16,
212-226.
|
 |
|
|
|
|
 |
J.J.Perry,
L.Fan,
and
J.A.Tainer
(2007).
Developing master keys to brain pathology, cancer and aging from the structural biology of proteins controlling reactive oxygen species and DNA repair.
|
| |
Neuroscience,
145,
1280-1299.
|
 |
|
|
|
|
 |
Y.H.Le Nguyen,
J.R.Winkler,
and
H.B.Gray
(2007).
Probing heme coordination states of inducible nitric oxide synthase with a ReI(imidazole-alkyl-nitroarginine) sensitizer-wire.
|
| |
J Phys Chem B,
111,
6628-6633.
|
 |
|
|
|
|
 |
L.E.Llewellyn
(2006).
Saxitoxin, a toxic marine natural product that targets a multitude of receptors.
|
| |
Nat Prod Rep,
23,
200-222.
|
 |
|
|
|
|
 |
R.Sengupta,
R.Sahoo,
S.S.Ray,
T.Dutta,
A.Dasgupta,
and
S.Ghosh
(2006).
Dissociation and unfolding of inducible nitric oxide synthase oxygenase domain identifies structural role of tetrahydrobiopterin in modulating the heme environment.
|
| |
Mol Cell Biochem,
284,
117-126.
|
 |
|
|
|
|
 |
T.L.Pukala,
J.H.Bowie,
V.M.Maselli,
I.F.Musgrave,
and
M.J.Tyler
(2006).
Host-defence peptides from the glandular secretions of amphibians: structure and activity.
|
| |
Nat Prod Rep,
23,
368-393.
|
 |
|
|
|
|
 |
D.J.Stuehr,
C.C.Wei,
Z.Wang,
and
R.Hille
(2005).
Exploring the redox reactions between heme and tetrahydrobiopterin in the nitric oxide synthases.
|
| |
Dalton Trans,
(),
3427-3435.
|
 |
|
|
|
|
 |
P.A.Loughran,
D.B.Stolz,
Y.Vodovotz,
S.C.Watkins,
R.L.Simmons,
and
T.R.Billiar
(2005).
Monomeric inducible nitric oxide synthase localizes to peroxisomes in hepatocytes.
|
| |
Proc Natl Acad Sci U S A,
102,
13837-13842.
|
 |
|
|
|
|
 |
H.Matter,
and
P.Kotsonis
(2004).
Biology and chemistry of the inhibition of nitric oxide synthases by pteridine-derivatives as therapeutic agents.
|
| |
Med Res Rev,
24,
662-684.
|
 |
|
|
|
|
 |
M.R.Buddha,
K.M.Keery,
and
B.R.Crane
(2004).
An unusual tryptophanyl tRNA synthetase interacts with nitric oxide synthase in Deinococcus radiodurans.
|
| |
Proc Natl Acad Sci U S A,
101,
15881-15886.
|
 |
|
|
|
|
 |
R.Fedorov,
R.Vasan,
D.K.Ghosh,
and
I.Schlichting
(2004).
Structures of nitric oxide synthase isoforms complexed with the inhibitor AR-R17477 suggest a rational basis for specificity and inhibitor design.
|
| |
Proc Natl Acad Sci U S A,
101,
5892-5897.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Ueda,
H.Terauchi,
M.Kawasaki,
A.Yano,
and
M.Ido
(2004).
Structure-activity relationships of 2-aminothiazole derivatives as inducible nitric oxide synthase inhibitor.
|
| |
Chem Pharm Bull (Tokyo),
52,
634-637.
|
 |
|
|
|
|
 |
A.C.Gorren,
K.Schmidt,
and
B.Mayer
(2002).
Binding of L-arginine and imidazole suggests heterogeneity of rat brain neuronal nitric oxide synthase.
|
| |
Biochemistry,
41,
7819-7829.
|
 |
|
|
|
|
 |
J.Doyle,
L.E.Llewellyn,
C.S.Brinkworth,
J.H.Bowie,
K.L.Wegener,
T.Rozek,
P.A.Wabnitz,
J.C.Wallace,
and
M.J.Tyler
(2002).
Amphibian peptides that inhibit neuronal nitric oxide synthase. Isolation of lesuerin from the skin secretion of the Australian Stony Creek frog Litoria lesueuri.
|
| |
Eur J Biochem,
269,
100-109.
|
 |
|
|
|
|
 |
J.P.Schelvis,
V.Berka,
G.T.Babcock,
and
A.L.Tsai
(2002).
Resonance Raman detection of the Fe-S bond in endothelial nitric oxide synthase.
|
| |
Biochemistry,
41,
5695-5701.
|
 |
|
|
|
|
 |
K.K.Wu
(2002).
Regulation of endothelial nitric oxide synthase activity and gene expression.
|
| |
Ann N Y Acad Sci,
962,
122-130.
|
 |
|
|
|
|
 |
K.Pant,
A.M.Bilwes,
S.Adak,
D.J.Stuehr,
and
B.R.Crane
(2002).
Structure of a nitric oxide synthase heme protein from Bacillus subtilis.
|
| |
Biochemistry,
41,
11071-11079.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Ascenzi,
M.Fasano,
M.Marino,
G.Venturini,
and
R.Federico
(2002).
Agmatine oxidation by copper amine oxidase.
|
| |
Eur J Biochem,
269,
884-892.
|
 |
|
|
|
|
 |
Y.Watanabe
(2002).
Construction of heme enzymes: four approaches.
|
| |
Curr Opin Chem Biol,
6,
208-216.
|
 |
|
|
|
|
 |
A.Reif,
L.Zecca,
P.Riederer,
M.Feelisch,
and
H.H.Schmidt
(2001).
Nitroxyl oxidizes NADPH in a superoxide dismutase inhibitable manner.
|
| |
Free Radic Biol Med,
30,
803-808.
|
 |
|
|
|
|
 |
D.K.Ghosh,
M.B.Rashid,
B.Crane,
V.Taskar,
M.Mast,
M.A.Misukonis,
J.B.Weinberg,
and
N.T.Eissa
(2001).
Characterization of key residues in the subdomain encoded by exons 8 and 9 of human inducible nitric oxide synthase: a critical role for Asp-280 in substrate binding and subunit interactions.
|
| |
Proc Natl Acad Sci U S A,
98,
10392-10397.
|
 |
|
|
|
|
 |
H.Jiang,
M.Ichikawa,
A.Furukawa,
S.Tomita,
T.Ohnishi,
and
Y.Ichikawa
(2001).
The optical interconversion of the P-450 and P-420 forms of neuronal nitric oxide synthase: effects of sodium cholate, mercury chloride and urea.
|
| |
Int J Biochem Cell Biol,
33,
155-162.
|
 |
|
|
|
|
 |
H.M.Abu-Soud,
K.Ichimori,
H.Nakazawa,
and
D.J.Stuehr
(2001).
Regulation of inducible nitric oxide synthase by self-generated NO.
|
| |
Biochemistry,
40,
6876-6881.
|
 |
|
|
|
|
 |
K.R.Wolthers,
and
M.I.Schimerlik
(2001).
Reaction of neuronal nitric-oxide synthase with 2,6-dichloroindolphenol and cytochrome c3+: influence of the electron acceptor and binding of Ca2+-activated calmodulin on the kinetic mechanism.
|
| |
Biochemistry,
40,
4722-4737.
|
 |
|
|
|
|
 |
A.W.Munro,
P.Taylor,
and
M.D.Walkinshaw
(2000).
Structures of redox enzymes.
|
| |
Curr Opin Biotechnol,
11,
369-376.
|
 |
|
|
|
|
 |
B.R.Crane,
A.S.Arvai,
S.Ghosh,
E.D.Getzoff,
D.J.Stuehr,
and
J.A.Tainer
(2000).
Structures of the N(omega)-hydroxy-L-arginine complex of inducible nitric oxide synthase oxygenase dimer with active and inactive pterins.
|
| |
Biochemistry,
39,
4608-4621.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.Jung,
D.J.Stuehr,
and
D.K.Ghosh
(2000).
FT-Infrared spectroscopic studies of the iron ligand CO stretch mode of iNOS oxygenase domain: effect of arginine and tetrahydrobiopterin.
|
| |
Biochemistry,
39,
10163-10171.
|
 |
|
|
|
|
 |
D.E.Danley,
M.E.Haggan,
D.Cunningham,
K.F.Fennell,
T.A.Pauly,
and
P.K.LeMotte
(2000).
A crystallizable form of RIIbeta regulatory domain obtained by limited proteolysis.
|
| |
Acta Crystallogr D Biol Crystallogr,
56,
1038-1041.
|
 |
|
|
|
|
 |
D.F.Lewis,
and
P.Hlavica
(2000).
Interactions between redox partners in various cytochrome P450 systems: functional and structural aspects.
|
| |
Biochim Biophys Acta,
1460,
353-374.
|
 |
|
|
|
|
 |
K.McMillan,
M.Adler,
D.S.Auld,
J.J.Baldwin,
E.Blasko,
L.J.Browne,
D.Chelsky,
D.Davey,
R.E.Dolle,
K.A.Eagen,
S.Erickson,
R.I.Feldman,
C.B.Glaser,
C.Mallari,
M.M.Morrissey,
M.H.Ohlmeyer,
G.Pan,
J.F.Parkinson,
G.B.Phillips,
M.A.Polokoff,
N.H.Sigal,
R.Vergona,
M.Whitlow,
T.A.Young,
and
J.J.Devlin
(2000).
Allosteric inhibitors of inducible nitric oxide synthase dimerization discovered via combinatorial chemistry.
|
| |
Proc Natl Acad Sci U S A,
97,
1506-1511.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Dall'Acqua,
and
P.Carter
(2000).
Substrate-assisted catalysis: molecular basis and biological significance.
|
| |
Protein Sci,
9,
1-9.
|
 |
|
|
|
|
 |
A.J.Hobbs,
A.Higgs,
and
S.Moncada
(1999).
Inhibition of nitric oxide synthase as a potential therapeutic target.
|
| |
Annu Rev Pharmacol Toxicol,
39,
191-220.
|
 |
|
|
|
|
 |
B.R.Crane,
R.J.Rosenfeld,
A.S.Arvai,
D.K.Ghosh,
S.Ghosh,
J.A.Tainer,
D.J.Stuehr,
and
E.D.Getzoff
(1999).
N-terminal domain swapping and metal ion binding in nitric oxide synthase dimerization.
|
| |
EMBO J,
18,
6271-6281.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.Tetreau,
M.Tourbez,
A.Gorren,
B.Mayer,
and
D.Lavalette
(1999).
Dynamics of carbon monoxide binding with neuronal nitric oxide synthase.
|
| |
Biochemistry,
38,
7210-7218.
|
 |
|
|
|
|
 |
D.J.Stuehr
(1999).
Mammalian nitric oxide synthases.
|
| |
Biochim Biophys Acta,
1411,
217-230.
|
 |
|
|
|
|
 |
D.K.Ghosh,
B.R.Crane,
S.Ghosh,
D.Wolan,
R.Gachhui,
C.Crooks,
A.Presta,
J.A.Tainer,
E.D.Getzoff,
and
D.J.Stuehr
(1999).
Inducible nitric oxide synthase: role of the N-terminal beta-hairpin hook and pterin-binding segment in dimerization and tetrahydrobiopterin interaction.
|
| |
EMBO J,
18,
6260-6270.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.Shelver,
M.V.Thorsteinsson,
R.L.Kerby,
S.Y.Chung,
G.P.Roberts,
M.F.Reynolds,
R.B.Parks,
and
J.N.Burstyn
(1999).
Identification of two important heme site residues (cysteine 75 and histidine 77) in CooA, the CO-sensing transcription factor of Rhodospirillum rubrum.
|
| |
Biochemistry,
38,
2669-2678.
|
 |
|
|
|
|
 |
J.L.Pellequer,
R.Brudler,
and
E.D.Getzoff
(1999).
Biological sensors: More than one way to sense oxygen.
|
| |
Curr Biol,
9,
R416-R418.
|
 |
|
|
|
|
 |
L.Huang,
H.M.Abu-Soud,
R.Hille,
and
D.J.Stuehr
(1999).
Nitric oxide-generated P420 nitric oxide synthase: characterization and roles for tetrahydrobiopterin and substrate in protecting against or reversing the P420 conversion.
|
| |
Biochemistry,
38,
1912-1920.
|
 |
|
|
|
|
 |
M.H.Vos,
and
J.L.Martin
(1999).
Femtosecond processes in proteins.
|
| |
Biochim Biophys Acta,
1411,
1.
|
 |
|
|
|
|
 |
O.G.Hallmark,
Y.T.Phung,
and
S.M.Black
(1999).
Chimeric forms of neuronal nitric oxide synthase identify different regions of the reductase domain that are essential for dimerization and activity.
|
| |
DNA Cell Biol,
18,
397-407.
|
 |
|
|
|
|
 |
P.Vallance,
and
A.Hingorani
(1999).
Endothelial nitric oxide in humans in health and disease.
|
| |
Int J Exp Pathol,
80,
291-303.
|
 |
|
|
|
|
 |
R.Gradini,
M.Realacci,
A.Ginepri,
G.Naso,
C.Santangelo,
O.Cela,
P.Sale,
A.Berardi,
E.Petrangeli,
M.Gallucci,
F.Di Silverio,
and
M.A.Russo
(1999).
Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology.
|
| |
J Pathol,
189,
224-229.
|
 |
|
|
|
|
 |
S.P.Fricker
(1999).
Nitric oxide scavengers as a therapeutic approach to nitric oxide mediated disease.
|
| |
Expert Opin Investig Drugs,
8,
1209-1222.
|
 |
|
|
|
|
 |
C.Moali,
J.L.Boucher,
M.A.Sari,
D.J.Stuehr,
and
D.Mansuy
(1998).
Substrate specificity of NO synthases: detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine.
|
| |
Biochemistry,
37,
10453-10460.
|
 |
|
|
|
|
 |
C.S.Raman,
H.Li,
P.Martásek,
V.Král,
B.S.Masters,
and
T.L.Poulos
(1998).
Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center.
|
| |
Cell,
95,
939-950.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.S.Bohle
(1998).
Pathophysiological chemistry of nitric oxide and its oxygenation by-products.
|
| |
Curr Opin Chem Biol,
2,
194-200.
|
 |
|
|
|
|
 |
H.M.Abu-Soud,
C.Wu,
D.K.Ghosh,
and
D.J.Stuehr
(1998).
Stopped-flow analysis of CO and NO binding to inducible nitric oxide synthase.
|
| |
Biochemistry,
37,
3777-3786.
|
 |
|
|
|
|
 |
J.M.Perry,
and
M.A.Marletta
(1998).
Effects of transition metals on nitric oxide synthase catalysis.
|
| |
Proc Natl Acad Sci U S A,
95,
11101-11106.
|
 |
|
|
|
|
 |
J.M.Perry,
N.Moon,
Y.Zhao,
W.R.Dunham,
and
M.A.Marletta
(1998).
The high-potential flavin and heme of nitric oxide synthase are not magnetically linked: implications for electron transfer.
|
| |
Chem Biol,
5,
355-364.
|
 |
|
|
|
|
 |
K.Brunner,
A.Tortschanoff,
B.Hemmens,
P.J.Andrew,
B.Mayer,
and
A.J.Kungl
(1998).
Sensitivity of flavin fluorescence dynamics in neuronal nitric oxide synthase to cofactor-induced conformational changes and dimerization.
|
| |
Biochemistry,
37,
17545-17553.
|
 |
|
|
|
|
 |
M.A.Marletta,
A.R.Hurshman,
and
K.M.Rusche
(1998).
Catalysis by nitric oxide synthase.
|
| |
Curr Opin Chem Biol,
2,
656-663.
|
 |
|
|
|
|
 |
N.T.Eissa,
J.W.Yuan,
C.M.Haggerty,
E.K.Choo,
C.D.Palmer,
and
J.Moss
(1998).
Cloning and characterization of human inducible nitric oxide synthase splice variants: a domain, encoded by exons 8 and 9, is critical for dimerization.
|
| |
Proc Natl Acad Sci U S A,
95,
7625-7630.
|
 |
|
|
|
|
 |
R.Cortesi,
P.Ascenzi,
M.Colasanti,
T.Persichini,
G.Venturini,
M.Bolognesi,
A.Pesce,
C.Nastruzzi,
and
E.Menegatti
(1998).
Cross-enzyme inhibition by gabexate mesylate: formulation and reactivity study.
|
| |
J Pharm Sci,
87,
1335-1340.
|
 |
|
|
|
|
 |
R.M.Clancy,
A.R.Amin,
and
S.B.Abramson
(1998).
The role of nitric oxide in inflammation and immunity.
|
| |
Arthritis Rheum,
41,
1141-1151.
|
 |
|
|
|
|
 |
T.L.Poulos,
C.S.Raman,
and
H.Li
(1998).
NO news is good news.
|
| |
Structure,
6,
255-258.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

