 |
PDBsum entry 6ykt
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.4
- leucine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Leu) + L-leucine + ATP = L-leucyl-tRNA(Leu) + AMP + diphosphate
|
 |
 |
 |
 |
 |
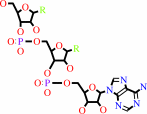 tRNA(Leu)
tRNA(Leu)
|
+
|
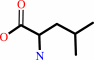 L-leucine
L-leucine
|
+
|
 ATP
ATP
|
=
|
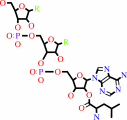 L-leucyl-tRNA(Leu)
L-leucyl-tRNA(Leu)
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Eur J Med Chem
211:113021-113021
(2021)
|
|
PubMed id:
|
|
|
|

|
| |
|
Synthesis and structure-activity studies of novel anhydrohexitol-based Leucyl-tRNA synthetase inhibitors.
|
|
D.De Ruysscher,
L.Pang,
S.M.G.Lenders,
D.Cappoen,
P.Cos,
J.Rozenski,
S.V.Strelkov,
S.D.Weeks,
A.Van Aerschot.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Leucyl-tRNA synthetase (LeuRS) is a clinically validated target for the
development of antimicrobials. This enzyme catalyzes the formation of charged
tRNALeu molecules, an essential substrate for protein translation. In
the first step of catalysis LeuRS activates leucine using ATP, forming a
leucyl-adenylate intermediate. Bi-substrate inhibitors that mimic this
chemically labile phosphoanhydride-linked nucleoside have proven to be potent
inhibitors of different members of the aminoacyl-tRNA synthetase family but, to
date, they have demonstrated poor antibacterial activity. We synthesized a small
series of 1,5-anhydrohexitol-based analogues coupled to a variety of triazoles
and performed detailed structure-activity relationship studies with bacterial
LeuRS. In an in vitro assay, Kiapp values in the
nanomolar range were demonstrated. Inhibitory activity differences between the
compounds revealed that the polarity and size of the triazole substituents
affect binding. X-ray crystallographic studies of N. gonorrhoeae LeuRS in
complex with all the inhibitors highlighted the crucial interactions defining
their relative enzyme inhibitory activities. We further examined their in vitro
antimicrobial properties by screening against several bacterial and yeast
strains. While only weak antibacterial activity against M. tuberculosis was
detected, the extensive structural data which were obtained could make these
LeuRS inhibitors a suitable starting point towards further antibiotic
development.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

