 |
PDBsum entry 6v2c
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Complex of mutant (k162m) of e. Coli l-asparaginase ii with l-asp. Covalent acyl-enzyme intermediate and tetrahedral intermediate
|
|
Structure:
|
 |
L-asparaginase 2. Chain: a, c, b, d. Synonym: l-asparaginase ii,l-asnase ii,l-asparagine amidohydrolase ii. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Escherichia coli (strain k12). Organism_taxid: 83333. Strain: k12. Gene: ansb, b2957, jw2924. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008. Expression_system_variant: jc2. Expression_system_cell: mesophilic bacteria.
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.173
|
R-free:
|
0.235
|
|
|
Authors:
|
 |
J.Lubkowski,A.Wlodawer
|
|
Key ref:
|
 |
J.Lubkowski
et al.
(2020).
Mechanism of Catalysis by l-Asparaginase.
Biochemistry,
59,
1927-1945.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
22-Nov-19
|
Release date:
|
20-May-20
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00805
(ASPG2_ECOLI) -
L-asparaginase 2 from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
348 a.a.
327 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 3 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.1.1
- asparaginase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-asparagine + H2O = L-aspartate + NH4+
|
 |
 |
 |
 |
 |
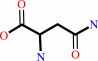 L-asparagine
L-asparagine
|
+
|
H2O
|
=
|
 L-aspartate
L-aspartate
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
59:1927-1945
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
Mechanism of Catalysis by l-Asparaginase.
|
|
J.Lubkowski,
J.Vanegas,
W.K.Chan,
P.L.Lorenzi,
J.N.Weinstein,
S.Sukharev,
D.Fushman,
S.Rempe,
A.Anishkin,
A.Wlodawer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Two bacterial type II l-asparaginases, from Escherichia coli and
Dickeya chrysanthemi, have played a critical role for more than 40 years
as therapeutic agents against juvenile leukemias and lymphomas. Despite a long
history of successful pharmacological applications and the apparent simplicity
of the catalytic reaction, controversies still exist regarding major steps of
the mechanism. In this report, we provide a detailed description of the reaction
catalyzed by E. coli type II l-asparaginase (EcAII). Our model was
developed on the basis of new structural and biochemical experiments combined
with previously published data. The proposed mechanism is supported by quantum
chemistry calculations based on density functional theory. We provide strong
evidence that EcAII catalyzes the reaction according to the double-displacement
(ping-pong) mechanism, with formation of a covalent intermediate. Several steps
of catalysis by EcAII are unique when compared to reactions catalyzed by other
known hydrolytic enzymes. Here, the reaction is initiated by a weak nucleophile,
threonine, without direct assistance of a general base, although a distant
general base is identified. Furthermore, tetrahedral intermediates formed during
the catalytic process are stabilized by a never previously described motif.
Although the scheme of the catalytic mechanism was developed only on the basis
of data obtained from EcAII and its variants, this novel mechanism of enzymatic
hydrolysis could potentially apply to most (and possibly all) l-asparaginases.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

