 |
PDBsum entry 6a0r

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
6a0r
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.1.1.3
- homoserine dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Threonine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
L-homoserine + NAD+ = L-aspartate 4-semialdehyde + NADH + H+
|
|
2.
|
L-homoserine + NADP+ = L-aspartate 4-semialdehyde + NADPH + H+
|
|
 |
 |
 |
 |
 |
L-homoserine
Bound ligand (Het Group name = )
matches with 40.00% similarity
|
+
|
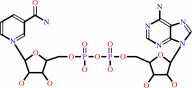 NAD(+)
NAD(+)
|
=
|
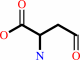 L-aspartate 4-semialdehyde
L-aspartate 4-semialdehyde
|
+
|
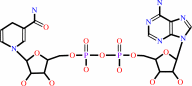 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
L-homoserine
Bound ligand (Het Group name = )
matches with 40.00% similarity
|
+
|
 NADP(+)
NADP(+)
|
=
|
 L-aspartate 4-semialdehyde
L-aspartate 4-semialdehyde
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biochem
165:185-195
(2019)
|
|
PubMed id:
|
|
|
|

|
| |
|
The crystal structure of homoserine dehydrogenase complexed with l-homoserine and NADPH in a closed form.
|
|
S.Akai,
H.Ikushiro,
T.Sawai,
T.Yano,
N.Kamiya,
I.Miyahara.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Homoserine dehydrogenase from Thermus thermophilus (TtHSD) is a key enzyme in
the aspartate pathway that catalyses the reversible conversion of
l-aspartate-β-semialdehyde to l-homoserine (l-Hse) with NAD(P)H. We determined
the crystal structures of unliganded TtHSD, TtHSD complexed with l-Hse and
NADPH, and Lys99Ala and Lys195Ala mutant TtHSDs, which have no enzymatic
activity, complexed with l-Hse and NADP+ at 1.83, 2.00, 1.87 and 1.93 Å
resolutions, respectively. Binding of l-Hse and NADPH induced the conformational
changes of TtHSD from an open to a closed form: the mobile loop containing
Glu180 approached to fix l-Hse and NADPH, and both Lys99 and Lys195 could make
hydrogen bonds with the hydroxy group of l-Hse. The ternary complex of TtHSDs in
the closed form mimicked a Michaelis complex better than the previously reported
open form structures from other species. In the crystal structure of Lys99Ala
TtHSD, the productive geometry of the ternary complex was almost preserved with
one new water molecule taking over the hydrogen bonds associated with Lys99,
while the positions of Lys195 and l-Hse were significantly retained with those
of the wild-type enzyme. These results propose new possibilities that Lys99 is
the acid-base catalytic residue of HSDs.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

