 |
PDBsum entry 5uvc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/transferase inhibitor
|
PDB id
|
|

|

|
5uvc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase/transferase inhibitor
|
 |
|
Title:
|
 |
Design, synthesis, and evaluation of the first selective and potent g- protein-coupled receptor kinase 2 (grk2) inhibitor for the potential treatment of heart failure
|
|
Structure:
|
 |
Beta-adrenergic receptor kinase 1. Chain: a. Fragment: unp residues 23-538. Synonym: beta-ark-1,g-protein coupled receptor kinase 2. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: grk2, adrbk1, bark, bark1. Expressed in: baculovirus expression vector pfastbac1-hm. Expression_system_taxid: 274590
|
|
Resolution:
|
 |
|
2.65Å
|
R-factor:
|
0.217
|
R-free:
|
0.260
|
|
|
Authors:
|
 |
I.D.Hoffman,J.D.Lawson
|
|
Key ref:
|
 |
T.Okawa
et al.
(2017).
Design, Synthesis, and Evaluation of the Highly Selective and Potent G-Protein-Coupled Receptor Kinase 2 (GRK2) Inhibitor for the Potential Treatment of Heart Failure.
J Med Chem,
60,
6942-6990.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
20-Feb-17
|
Release date:
|
26-Jul-17
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P25098
(ARBK1_HUMAN) -
Beta-adrenergic receptor kinase 1 from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
689 a.a.
457 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.11.15
- [beta-adrenergic-receptor] kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
[beta-adrenergic receptor] + ATP = [beta-adrenergic receptor]-phosphate + ADP + H+
|
 |
 |
 |
 |
 |
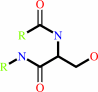 [beta-adrenergic receptor]
[beta-adrenergic receptor]
|
+
|
 ATP
ATP
|
=
|
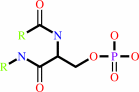 [beta-adrenergic receptor]-phosphate
[beta-adrenergic receptor]-phosphate
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
60:6942-6990
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Design, Synthesis, and Evaluation of the Highly Selective and Potent G-Protein-Coupled Receptor Kinase 2 (GRK2) Inhibitor for the Potential Treatment of Heart Failure.
|
|
T.Okawa,
Y.Aramaki,
M.Yamamoto,
T.Kobayashi,
S.Fukumoto,
Y.Toyoda,
T.Henta,
A.Hata,
S.Ikeda,
M.Kaneko,
I.D.Hoffman,
B.C.Sang,
H.Zou,
T.Kawamoto.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A novel class of therapeutic drug candidates for heart failure, highly potent
and selective GRK2 inhibitors, exhibit potentiation of β-adrenergic signaling
in vitro studies. Hydrazone derivative 5 and 1,2,4-triazole derivative 24a were
identified as hit compounds by HTS. New scaffold generation and SAR studies of
all parts resulted in a 4-methyl-1,2,4-triazole derivative with an
N-benzylcarboxamide moiety with highly potent activity toward GRK2 and
selectivity over other kinases. In terms of subtype selectivity, these compounds
showed enough selectivity against GRK1, 5, 6, and 7 with almost equipotent
inhibition to GRK3. Our medicinal chemistry efforts led to the discovery of 115h
(GRK2 IC50= 18 nM), which was obtained the cocrystal structure with
human GRK2 and an inhibitor of GRK2 that potentiates β-adrenergic receptor
(βAR)-mediated cAMP accumulation and prevents internalization of βARs in
β2AR-expressing HEK293 cells treated with isoproterenol. Therefore, 115h
appears to be a novel class of therapeutic for heart failure treatment.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

