 |
PDBsum entry 5ll3
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.1.1.21
- isoleucine 2-epimerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-isoleucine = D-allo-isoleucine
|
 |
 |
 |
 |
 |
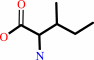 L-isoleucine
L-isoleucine
|
=
|
D-allo-isoleucine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochimie
137:165-173
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural insights into the substrate recognition and reaction specificity of the PLP-dependent fold-type I isoleucine 2-epimerase from Lactobacillus buchneri.
|
|
R.Awad,
P.Gans,
J.B.Reiser.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The isoleucine 2-epimerase from Lactobacillus buchneri has been previously
identified and characterized to catalyze the pyridoxal 5'-phosphate
(PLP)-dependent racemization and epimerization of a broad spectrum of nonpolar
amino acids from L- to D-form and vice versa, in particular isoleucine. In this
study, crystal structures of both native and PLP-complex forms of this racemase
are presented at 2.6 and 2.15 Å resolution, respectively. Both structures show
that the protein belongs to the fold-type I subgroup of PLP-dependent enzymes
and is very close to aminobutyrate aminotransferases family, as it has been
suspected because of their sequence homology. The extensive structural
comparison with fold-type I enzymes with known amino acid racemization
activities, including the α-amino-ε-caprolactam racemase from Achromobacter
obae and the cystathionine β-lyase from Escherichia coli, allows us to identify
the active site residues responsible for its nonpolar amino acid recognition and
reactivity specificity. Our observations also suggest that the racemization
reaction by the fold-type I racemases may generally occur thanks to a revised
two-base mechanism. Lastly, both structures reveal details on the conformational
changes provoked by PLP binding that suggest an induced fit of the active site
"entrance door", necessary to accommodate PLP and substrate molecules.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

