 |
PDBsum entry 5u99
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.2.17
- Atp phosphoribosyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Histidine Biosynthesis (early stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
1-(5-phospho-beta-D-ribosyl)-ATP + diphosphate = 5-phospho-alpha-D-ribose 1-diphosphate + ATP
|
 |
 |
 |
 |
 |
1-(5-phospho-beta-D-ribosyl)-ATP
|
+
|
 diphosphate
diphosphate
|
=
|
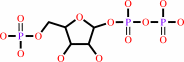 5-phospho-alpha-D-ribose 1-diphosphate
5-phospho-alpha-D-ribose 1-diphosphate
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
ACS Chem Biol
12:2662-2670
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Transition State Analysis of Adenosine Triphosphate Phosphoribosyltransferase.
|
|
G.J.Moggré,
M.B.Poulin,
P.C.Tyler,
V.L.Schramm,
E.J.Parker.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Adenosine triphosphate phosphoribosyltransferase (ATP-PRT) catalyzes the first
step in histidine biosynthesis, a pathway essential to microorganisms and a
validated target for antimicrobial drug design. The ATP-PRT enzyme catalyzes the
reversible substitution reaction between phosphoribosyl pyrophosphate and ATP.
The enzyme exists in two structurally distinct forms, a short- and a long-form
enzyme. These forms share a catalytic core dimer but bear completely different
allosteric domains and thus distinct quaternary assemblies. Understanding
enzymatic transition states can provide essential information on the reaction
mechanisms and insight into how differences in domain structure influence the
reaction chemistry, as well as providing a template for inhibitor design. In
this study, the transition state structures for ATP-PRT enzymes from
Campylobacter jejuni and Mycobacterium tuberculosis (long-form enzymes) and from
Lactococcus lactis (short-form) were determined and compared. Intrinsic kinetic
isotope effects (KIEs) were obtained at reaction sensitive positions for the
reverse reaction using phosphonoacetic acid, an alternative substrate to the
natural substrate pyrophosphate. The experimental KIEs demonstrated mechanistic
similarities between the three enzymes and provided experimental boundaries for
quantum chemical calculations to characterize the transition states. Predicted
transition state structures support a dissociative reaction mechanism with a
DN*AN‡transition state. Weak interactions
from the incoming nucleophile and a fully dissociated ATP adenine are predicted
regardless of the difference in overall structure and quaternary assembly. These
studies establish that despite significant differences in the quaternary
assembly and regulatory machinery between ATP-PRT enzymes from different
sources, the reaction chemistry and catalytic mechanism are conserved.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

