 |
PDBsum entry 5g5e
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.5.1.18
- glutathione transferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RX + glutathione = an S-substituted glutathione + a halide anion + H+
|
 |
 |
 |
 |
 |
 RX
RX
|
+
|
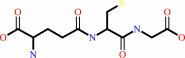 glutathione
glutathione
|
=
|
S-substituted glutathione
|
+
|
halide anion
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochimie
135:35-45
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Insights into ligand binding to a glutathione S-transferase from mango: Structure, thermodynamics and kinetics.
|
|
I.Valenzuela-Chavira,
C.A.Contreras-Vergara,
A.A.Arvizu-Flores,
H.Serrano-Posada,
A.A.Lopez-Zavala,
K.D.García-Orozco,
J.Hernandez-Paredes,
E.Rudiño-Piñera,
V.Stojanoff,
R.R.Sotelo-Mundo,
M.A.Islas-Osuna.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
We studied a mango glutathione S-transferase (GST) (Mangifera indica) bound to
glutathione (GSH) and S-hexyl glutathione (GSX). This GST Tau class (MiGSTU) had
a molecular mass of 25.5 kDa. MiGSTU Michaelis-Menten kinetic constants were
determined for their substrates obtaining a Km, Vmax and kcat for CDNB of
0.792 mM, 80.58 mM min(-1) and 68.49 s(-1) respectively and 0.693 mM,
105.32 mM min(-1) and 89.57 s(-1), for reduced GSH respectively. MiGSTU had a
micromolar affinity towards GSH (5.2 μM) or GSX (7.8 μM). The crystal
structure of the MiGSTU in apo or bound to GSH or GSX generated a model that
explains the thermodynamic signatures of binding and showed the importance of
enthalpic-entropic compensation in ligand binding to Tau-class GST enzymes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

