 |
PDBsum entry 5cts

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxo-acid-lyase
|
PDB id
|
|

|

|
5cts
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.3.1
- citrate (Si)-synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
oxaloacetate + acetyl-CoA + H2O = citrate + CoA + H+
|
 |
 |
 |
 |
 |
oxaloacetate
Bound ligand (Het Group name = )
matches with 94.34% similarity
|
+
|
 acetyl-CoA
acetyl-CoA
|
+
|
H2O
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
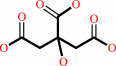 citrate
citrate
|
+
|
 CoA
CoA
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
29:2213-2219
(1990)
|
|
PubMed id:
|
|
|
|

|
| |
|
Proposed mechanism for the condensation reaction of citrate synthase: 1.9-A structure of the ternary complex with oxaloacetate and carboxymethyl coenzyme A.
|
|
M.Karpusas,
B.Branchaud,
S.J.Remington.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of the ternary complex citrate
synthase-oxaloacetate-carboxymethyl coenzyme A has been solved to a resolution
of 1.9 A and refined to a conventional crystallographic R factor of 0.185. The
structure resembles a proposed transition state of the condensation reaction and
suggests that the condensation reaction proceeds through a neutral enol rather
than an enolate intermediate. A mechanism for the condensation reaction is
proposed which involves the participation of three key catalytic groups (Asp
375, His 274, and His 320) in two distinct steps. The proposed mechanism invokes
concerted general acid-base catalysis twice to explain both the energetics of
the reaction and the experimentally observed inversion of stereochemistry at the
attacking carbon atom.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.Chittori,
H.S.Savithri,
and
M.R.Murthy
(2011).
Crystal structure of Salmonella typhimurium 2-methylcitrate synthase: Insights on domain movement and substrate specificity.
|
| |
J Struct Biol,
174,
58-68.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Tripathi,
and
G.E.Kellogg
(2010).
A novel and efficient tool for locating and characterizing protein cavities and binding sites.
|
| |
Proteins,
78,
825-842.
|
 |
|
|
|
|
 |
R.Lonsdale,
K.E.Ranaghan,
and
A.J.Mulholland
(2010).
Computational enzymology.
|
| |
Chem Commun (Camb),
46,
2354-2372.
|
 |
|
|
|
|
 |
L.C.Kurz,
C.Z.Constantine,
H.Jiang,
and
T.J.Kappock
(2009).
The partial substrate dethiaacetyl-coenzyme A mimics all critical carbon acid reactions in the condensation half-reaction catalyzed by Thermoplasma acidophilum citrate synthase.
|
| |
Biochemistry,
48,
7878-7891.
|
 |
|
|
|
|
 |
S.L.Bulfer,
E.M.Scott,
J.F.Couture,
L.Pillus,
and
R.C.Trievel
(2009).
Crystal structure and functional analysis of homocitrate synthase, an essential enzyme in lysine biosynthesis.
|
| |
J Biol Chem,
284,
35769-35780.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.L.Cheng,
C.C.Liao,
W.H.Tsai,
C.C.Lin,
C.W.Yeh,
C.F.Teng,
and
W.T.Chang
(2009).
Identification and characterization of the mitochondrial targeting sequence and mechanism in human citrate synthase.
|
| |
J Cell Biochem,
107,
1002-1015.
|
 |
|
|
|
|
 |
M.W.van der Kamp,
and
A.J.Mulholland
(2008).
Computational enzymology: insight into biological catalysts from modelling.
|
| |
Nat Prod Rep,
25,
1001-1014.
|
 |
|
|
|
|
 |
M.W.van der Kamp,
F.Perruccio,
and
A.J.Mulholland
(2008).
High-level QM/MM modelling predicts an arginine as the acid in the condensation reaction catalysed by citrate synthase.
|
| |
Chem Commun (Camb),
(),
1874-1876.
|
 |
|
|
|
|
 |
S.Friedmann,
B.E.Alber,
and
G.Fuchs
(2007).
Properties of R-citramalyl-coenzyme A lyase and its role in the autotrophic 3-hydroxypropionate cycle of Chloroflexus aurantiacus.
|
| |
J Bacteriol,
189,
2906-2914.
|
 |
|
|
|
|
 |
M.Ikeguchi,
J.Ueno,
M.Sato,
and
A.Kidera
(2005).
Protein structural change upon ligand binding: linear response theory.
|
| |
Phys Rev Lett,
94,
078102.
|
 |
|
|
|
|
 |
M.Suksomtip,
P.Liu,
T.Anderson,
S.Tungpradabkul,
D.W.Wood,
and
E.W.Nester
(2005).
Citrate synthase mutants of Agrobacterium are attenuated in virulence and display reduced vir gene induction.
|
| |
J Bacteriol,
187,
4844-4852.
|
 |
|
|
|
|
 |
Y.C.Huang,
Y.H.Chen,
S.R.Lo,
C.I.Liu,
C.W.Wang,
and
W.T.Chang
(2004).
Disruption of the peroxisomal citrate synthase CshA affects cell growth and multicellular development in Dictyostelium discoideum.
|
| |
Mol Microbiol,
53,
81-91.
|
 |
|
|
|
|
 |
C.V.Smith,
C.C.Huang,
A.Miczak,
D.G.Russell,
J.C.Sacchettini,
and
K.Höner zu Bentrup
(2003).
Biochemical and structural studies of malate synthase from Mycobacterium tuberculosis.
|
| |
J Biol Chem,
278,
1735-1743.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.M.Anstrom,
K.Kallio,
and
S.J.Remington
(2003).
Structure of the Escherichia coli malate synthase G:pyruvate:acetyl-coenzyme A abortive ternary complex at 1.95 A resolution.
|
| |
Protein Sci,
12,
1822-1832.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Severcan,
and
P.I.Haris
(2003).
Fourier transform infrared spectroscopy suggests unfolding of loop structures precedes complete unfolding of pig citrate synthase.
|
| |
Biopolymers,
69,
440-447.
|
 |
|
|
|
|
 |
G.Ruprich-Robert,
D.Zickler,
V.Berteaux-Lecellier,
C.Vélot,
and
M.Picard
(2002).
Lack of mitochondrial citrate synthase discloses a new meiotic checkpoint in a strict aerobe.
|
| |
EMBO J,
21,
6440-6451.
|
 |
|
|
|
|
 |
B.R.Howard,
J.A.Endrizzi,
and
S.J.Remington
(2000).
Crystal structure of Escherichia coli malate synthase G complexed with magnesium and glyoxylate at 2.0 A resolution: mechanistic implications.
|
| |
Biochemistry,
39,
3156-3168.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.D.Clark,
J.R.Allen,
and
S.A.Ensign
(2000).
Characterization of five catalytic activities associated with the NADPH:2-ketopropyl-coenzyme M [2-(2-ketopropylthio)ethanesulfonate] oxidoreductase/carboxylase of the Xanthobacter strain Py2 epoxide carboxylase system.
|
| |
Biochemistry,
39,
1294-1304.
|
 |
|
|
|
|
 |
I.P.Petrounia,
G.Blotny,
and
R.M.Pollack
(2000).
Binding of 2-naphthols to D38E mutants of 3-oxo-Delta 5-steroid isomerase: variation of ligand ionization state with the nature of the electrophilic component.
|
| |
Biochemistry,
39,
110-116.
|
 |
|
|
|
|
 |
L.C.Kurz,
G.Drysdale,
M.Riley,
M.A.Tomar,
J.Chen,
R.J.Russell,
and
M.J.Danson
(2000).
Kinetics and mechanism of the citrate synthase from the thermophilic archaeon Thermoplasma acidophilum.
|
| |
Biochemistry,
39,
2283-2296.
|
 |
|
|
|
|
 |
Z.Gu,
D.G.Drueckhammer,
L.Kurz,
K.Liu,
D.P.Martin,
and
A.McDermott
(1999).
Solid state NMR studies of hydrogen bonding in a citrate synthase inhibitor complex.
|
| |
Biochemistry,
38,
8022-8031.
|
 |
|
|
|
|
 |
G.Zhou,
T.Somasundaram,
E.Blanc,
G.Parthasarathy,
W.R.Ellington,
and
M.S.Chapman
(1998).
Transition state structure of arginine kinase: implications for catalysis of bimolecular reactions.
|
| |
Proc Natl Acad Sci U S A,
95,
8449-8454.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Grallert,
K.Rutkat,
and
J.Buchner
(1998).
GroEL traps dimeric and monomeric unfolding intermediates of citrate synthase.
|
| |
J Biol Chem,
273,
33305-33310.
|
 |
|
|
|
|
 |
L.C.Kurz,
T.Nakra,
R.Stein,
W.Plungkhen,
M.Riley,
F.Hsu,
and
G.R.Drysdale
(1998).
Effects of changes in three catalytic residues on the relative stabilities of some of the intermediates and transition states in the citrate synthase reaction.
|
| |
Biochemistry,
37,
9724-9737.
|
 |
|
|
|
|
 |
C.L.Perrin,
and
J.B.Nielson
(1997).
"Strong" hydrogen bonds in chemistry and biology.
|
| |
Annu Rev Phys Chem,
48,
511-544.
|
 |
|
|
|
|
 |
L.C.Kurz,
J.H.Roble,
T.Nakra,
G.R.Drysdale,
J.M.Buzan,
B.Schwartz,
and
D.G.Drueckhammer
(1997).
Ability of single-site mutants of citrate synthase to catalyze proton transfer from the methyl group of dethiaacetyl-coenzyme A, a non-thioester substrate analog.
|
| |
Biochemistry,
36,
3981-3990.
|
 |
|
|
|
|
 |
C.T.Evans,
L.C.Kurz,
S.J.Remington,
and
P.A.Srere
(1996).
Active site mutants of pig citrate synthase: effects of mutations on the enzyme catalytic and structural properties.
|
| |
Biochemistry,
35,
10661-10672.
|
 |
|
|
|
|
 |
I.Misra,
and
H.M.Miziorko
(1996).
Evidence for the interaction of avian 3-hydroxy-3-methylglutaryl-CoA synthase histidine 264 with acetoacetyl-CoA.
|
| |
Biochemistry,
35,
9610-9616.
|
 |
|
|
|
|
 |
J.L.Markley,
and
W.M.Westler
(1996).
Protonation-state dependence of hydrogen bond strengths and exchange rates in a serine protease catalytic triad: bovine chymotrypsinogen A.
|
| |
Biochemistry,
35,
11092-11097.
|
 |
|
|
|
|
 |
U.Jakob,
H.Lilie,
I.Meyer,
and
J.Buchner
(1995).
Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo.
|
| |
J Biol Chem,
270,
7288-7294.
|
 |
|
|
|
|
 |
A.J.Patton,
D.W.Hough,
P.Towner,
and
M.J.Danson
(1993).
Does Escherichia coli possess a second citrate synthase gene?
|
| |
Eur J Biochem,
214,
75-81.
|
 |
|
|
|
|
 |
M.A.Ech-Cherif el-Kettani,
K.Zakrzewska,
J.Durup,
and
R.Lavery
(1993).
An analysis of the conformational paths of citrate synthase.
|
| |
Proteins,
16,
393-407.
|
 |
|
|
|
|
 |
M.A.Ech-Cherif el-Kettani,
and
J.Durup
(1992).
Theoretical determination of conformational paths in citrate synthase.
|
| |
Biopolymers,
32,
561-574.
|
 |
|
|
|
|
 |
U.Lill,
S.Lefrank,
A.Henschen,
and
H.Eggerer
(1992).
Conversion, by limited proteolysis, of an archaebacterial citrate synthase into essentially a citryl-CoA hydrolase.
|
| |
Eur J Biochem,
208,
459-466.
|
 |
|
|
|
|
 |
U.Lill,
A.Kollmann-Koch,
A.Bibinger,
and
H.Eggerer
(1991).
Inhibitors of metabolic reactions. Scope and limitation of acyl-CoA-analogue CoA-thioethers.
|
| |
Eur J Biochem,
198,
767-773.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

