 |
PDBsum entry 4ztb
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.7.7.19
- polynucleotide adenylyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + ATP = RNA(n)-3'-adenine ribonucleotide + diphosphate
|
 |
 |
 |
 |
 |
 RNA(n)
RNA(n)
|
+
|
 ATP
ATP
|
=
|
RNA(n)-3'-adenine ribonucleotide
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.1.3.84
- ADP-ribose 1''-phosphate phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ADP-alpha-D-ribose 1''-phosphate + H2O = ADP-D-ribose + phosphate
|
 |
 |
 |
 |
 |
ADP-alpha-D-ribose 1''-phosphate
|
+
|
H2O
|
=
|
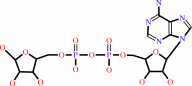 ADP-D-ribose
ADP-D-ribose
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.6.1.15
- nucleoside-triphosphate phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-triphosphate + H2O = a ribonucleoside 5'-diphosphate + phosphate + H+
|
 |
 |
 |
 |
 |
ribonucleoside 5'-triphosphate
|
+
|
H2O
|
=
|
ribonucleoside 5'-diphosphate
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.3.6.1.74
- mRNA 5'-phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end triphospho-ribonucleoside in mRNA + H2O = a 5'-end diphospho- ribonucleoside in mRNA + phosphate + H+
|
 |
 |
 |
 |
 |
5'-end triphospho-ribonucleoside in mRNA
|
+
|
H2O
|
=
|
5'-end diphospho- ribonucleoside in mRNA
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Int J Biol Macromol
116:451-462
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of chikungunya virus nsP2 cysteine protease reveals a putative flexible loop blocking its active site.
|
|
M.Narwal,
H.Singh,
S.Pratap,
A.Malik,
R.J.Kuhn,
P.Kumar,
S.Tomar.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Chikungunya virus (CHIKV), a mosquito-borne pathogenic alphavirus is a growing
public health threat. No vaccines or antiviral drug is currently available in
the market for chikungunya treatment. nsP2pro, the viral cysteine protease,
carries out an essential function of nonstructural polyprotein processing and
forms four nonstructural proteins (nsPs) that makes the replication complex,
hence constitute a promising drug target. In this study, crystal structure of
nsP2pro has been determined at 2.59 Å, which reveals that the protein
consists of two subdomains: an N-terminal protease subdomain and a C-terminal
methyltransferase subdomain. Structural comparison of CHIKV nsP2pro with
structures of other alphavirus nsP2 advances that the substrate binding cleft is
present at the interface of two subdomains. Additionally, structure insights
revealed that access to the active site and substrate binding cleft is blocked
by a flexible interdomain loop in CHIKV nsP2pro. This loop contains His548, the
catalytic residue, and Trp549 and Asn547, the residues predicted to bind
substrate. Interestingly, mutation of Asn547 leads to three-fold increase in
Km confirming that Asn547 plays important role in substrate binding
and recognition. This study presents the detailed molecular analysis and
signifies the substrate specificity residues of CHIKV nsP2pro, which will be
beneficial for structure-based drug design and optimization of CHIKV protease
inhibitors.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

