 |
PDBsum entry 4nfi

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/transferase inhibitor
|
PDB id
|
|

|

|
4nfi
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase/transferase inhibitor
|
 |
|
Title:
|
 |
Crystal structure of human fpps in complex with magnesium and jds05120
|
|
Structure:
|
 |
Farnesyl pyrophosphate synthase. Chain: f. Synonym: fpp synthase, fps, (2e,6e)-farnesyl diphosphate synthase, dimethylallyltranstransferase, farnesyl diphosphate synthase, geranyltranstransferase. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: fdps, fps, kiaa1293. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
1.85Å
|
R-factor:
|
0.166
|
R-free:
|
0.196
|
|
|
Authors:
|
 |
J.Park,J.W.De Schutter,Y.S.Tsantrizos,A.M.Berghuis
|
|
Key ref:
|
 |
J.Park
et al.
(2017).
Crystallographic and thermodynamic characterization of phenylaminopyridine bisphosphonates binding to human farnesyl pyrophosphate synthase.
PLoS One,
12,
e0186447.
PubMed id:
|
 |
|
Date:
|
 |
|
31-Oct-13
|
Release date:
|
31-Dec-14
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P14324
(FPPS_HUMAN) -
Farnesyl pyrophosphate synthase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
419 a.a.
344 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.5.1.1
- dimethylallyltranstransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Terpenoid biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + dimethylallyl diphosphate = (2E)- geranyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
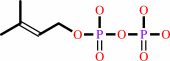 dimethylallyl diphosphate
dimethylallyl diphosphate
|
=
|
(2E)- geranyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.5.1.10
- (2E,6E)-farnesyl diphosphate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + (2E)-geranyl diphosphate = (2E,6E)-farnesyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
(2E)-geranyl diphosphate
|
=
|
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
PLoS One
12:e0186447
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystallographic and thermodynamic characterization of phenylaminopyridine bisphosphonates binding to human farnesyl pyrophosphate synthase.
|
|
J.Park,
D.Rodionov,
J.W.De Schutter,
Y.S.Lin,
Y.S.Tsantrizos,
A.M.Berghuis.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Human farnesyl pyrophosphate synthase (hFPPS) catalyzes the production of the
15-carbon isoprenoid farnesyl pyrophosphate. The enzyme is a key regulator of
the mevalonate pathway and a well-established drug target. Notably, it was
elucidated as the molecular target of nitrogen-containing bisphosphonates, a
class of drugs that have been widely successful against bone resorption
disorders. More recently, research has focused on the anticancer effects of
these inhibitors. In order to achieve increased non-skeletal tissue exposure, we
created phenylaminopyridine bisphosphonates (PNP-BPs) that have bulky
hydrophobic side chains through a structure-based approach. Some of these
compounds have proven to be more potent than the current clinical drugs in a
number of antiproliferation assays using multiple myeloma cell lines. In the
present work, we characterized the binding of our most potent PNP-BPs to the
target enzyme, hFPPS. Co-crystal structures demonstrate that the molecular
interactions designed to elicit tighter binding are indeed established. We
carried out thermodynamic studies as well; the newly introduced protein-ligand
interactions are clearly reflected in the enthalpy of binding measured, which is
more favorable for the new PNP-BPs than for the lead compound. These studies
also indicate that the affinity of the PNP-BPs to hFPPS is comparable to that of
the current drug risedronate. Risedronate forms additional polar interactions
via its hydroxyl functional group and thus exhibits more favorable binding
enthalpy; however, the entropy of binding is more favorable for the PNP-BPs,
owing to the greater desolvation effects resulting from their large hydrophobic
side chains. These results therefore confirm the overall validity of our drug
design strategy. With a distinctly different molecular scaffold, the PNP-BPs
described in this report represent an interesting new group of future drug
candidates. Further investigation should follow to characterize the tissue
distribution profile and assess the potential clinical benefits of these
compounds.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

