 |
PDBsum entry 3vtk

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.1.21
- thymidine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
thymidine + ATP = dTMP + ADP + H+
|
 |
 |
 |
 |
 |
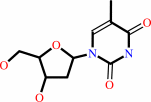 thymidine
thymidine
|
+
|
 ATP
ATP
|
=
|
dTMP
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
ADP
Bound ligand (Het Group name = )
matches with 90.91% similarity
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Protein Sci
6:2097-2106
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structures of thymidine kinase from herpes simplex virus type 1 in complex with substrates and a substrate analogue.
|
|
K.Wild,
T.Bohner,
G.Folkers,
G.E.Schulz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Thymidine kinase from Herpes simplex virus type 1 (TK) was crystallized in an
N-terminally truncated but fully active form. The structures of TK complexed
with ADP at the ATP-site and deoxythymidine-5'-monophosphate (dTMP),
deoxythymidine (dT), or idoxuridine-5'-phosphate (5-iodo-dUMP) at the
substrate-site were refined to 2.75 A, 2.8 A, and 3.0 A resolution,
respectively. TK catalyzes the phosphorylation of dT resulting in an ester, and
the phosphorylation of dTMP giving rise to an anhydride. The presented TK
structures indicate that there are only small differences between these two
modes of action. Glu83 serves as a general base in the ester reaction. Arg163
parks at an internal aspartate during ester formation and binds the
alpha-phosphate of dTMP during anhydride formation. The bound deoxythymidine
leaves a 35 A3 cavity at position 5 of the base and two sequestered water
molecules at position 2. Cavity and water molecules reduce the substrate
specificity to such an extent that TK can phosphorylate various substrate
analogues useful in pharmaceutical applications. TK is structurally homologous
to the well-known nucleoside monophosphate kinases but contains large additional
peptide segments.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
ig. 1. Stereoviewofthesymmetric TK dimerwithboundADPanddTMP.Thedomainsaredefinedaccording to theNMP-kinases
asCORE(residues 46-81,143-218.227-250.323-376, yellow, Wbi n,j (82-142, red), and ID (219-226, blue).The 72 additional
residuesaroundosition 90 are green.Mobileparts are givenasdashedlines,thetwofold axis is inserted,secondary structures are
defined in Fig. 2.
|
 |
Figure 3.
Fig. 3. Final (2F0 - F,)-electron density maps or all substrates with the C trace of the P-loop as reference. All maps are contoured
at the .0 (T level. A: ADPanddTPat 8, B: ADP and dTat 2.8 8, resolution. C: ADPand at 3.0 8,
resolution.
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by the Protein Society:
Protein Sci
(1997,
6,
2097-2106)
copyright 1997.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.C.Hsu,
Y.F.Chen,
S.R.Lin,
and
J.M.Yang
(2011).
iGEMDOCK: a graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis.
|
| |
BMC Bioinformatics,
12,
S33.
|
 |
|
|
|
|
 |
P.W.Krug,
R.F.Schinazi,
and
J.K.Hilliard
(2010).
Inhibition of B virus (Macacine herpesvirus 1) by conventional and experimental antiviral compounds.
|
| |
Antimicrob Agents Chemother,
54,
452-459.
|
 |
|
|
|
|
 |
Y.F.Chen,
K.C.Hsu,
S.R.Lin,
W.C.Wang,
Y.C.Huang,
and
J.M.Yang
(2010).
SiMMap: a web server for inferring site-moiety map to recognize interaction preferences between protein pockets and compound moieties.
|
| |
Nucleic Acids Res,
38,
W424-W430.
|
 |
|
|
|
|
 |
C.Caillat,
D.Topalis,
L.A.Agrofoglio,
S.Pochet,
J.Balzarini,
D.Deville-Bonne,
and
P.Meyer
(2008).
Crystal structure of poxvirus thymidylate kinase: an unexpected dimerization has implications for antiviral therapy.
|
| |
Proc Natl Acad Sci U S A,
105,
16900-16905.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.T.Hussein,
R.N.Miguel,
L.S.Tiley,
and
H.J.Field
(2008).
Substrate specificity and molecular modelling of the feline herpesvirus-1 thymidine kinase.
|
| |
Arch Virol,
153,
495-505.
|
 |
|
|
|
|
 |
M.J.Pérez-Pérez,
E.M.Priego,
A.I.Hernández,
O.Familiar,
M.J.Camarasa,
A.Negri,
F.Gago,
and
J.Balzarini
(2008).
Structure, physiological role, and specific inhibitors of human thymidine kinase 2 (TK2): present and future.
|
| |
Med Res Rev,
28,
797-820.
|
 |
|
|
|
|
 |
C.Luo,
A.Nawa,
Y.Yamauchi,
S.Kohno,
Y.Ushijima,
F.Goshima,
F.Kikkawa,
and
Y.Nishiyama
(2007).
Intercellular trafficking and cytotoxicity of recombinant HSV-1 thymidine kinase fused with HSV-2 US11 RXP repeat peptide.
|
| |
Virus Genes,
34,
263-272.
|
 |
|
|
|
|
 |
L.Egeblad-Welin,
Y.Sonntag,
H.Eklund,
and
B.Munch-Petersen
(2007).
Functional studies of active-site mutants from Drosophila melanogaster deoxyribonucleoside kinase. Investigations of the putative catalytic glutamate-arginine pair and of residues responsible for substrate specificity.
|
| |
FEBS J,
274,
1542-1551.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.I.Besecker,
C.L.Furness,
D.M.Coen,
and
A.Griffiths
(2007).
Expression of extremely low levels of thymidine kinase from an acyclovir-resistant herpes simplex virus mutant supports reactivation from latently infected mouse trigeminal ganglia.
|
| |
J Virol,
81,
8356-8360.
|
 |
|
|
|
|
 |
A.Griffiths,
M.A.Link,
C.L.Furness,
and
D.M.Coen
(2006).
Low-level expression and reversion both contribute to reactivation of herpes simplex virus drug-resistant mutants with mutations on homopolymeric sequences in thymidine kinase.
|
| |
J Virol,
80,
6568-6574.
|
 |
|
|
|
|
 |
K.El Omari,
N.Solaroli,
A.Karlsson,
J.Balzarini,
and
D.K.Stammers
(2006).
Structure of vaccinia virus thymidine kinase in complex with dTTP: insights for drug design.
|
| |
BMC Struct Biol,
6,
22.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Zhang,
J.A.Secrist,
and
S.E.Ealick
(2006).
The structure of human deoxycytidine kinase in complex with clofarabine reveals key interactions for prodrug activation.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
133-139.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Kontoyianni,
G.S.Sokol,
and
L.M.McClellan
(2005).
Evaluation of library ranking efficacy in virtual screening.
|
| |
J Comput Chem,
26,
11-22.
|
 |
|
|
|
|
 |
M.Welin,
T.Skovgaard,
W.Knecht,
C.Zhu,
D.Berenstein,
B.Munch-Petersen,
J.Piskur,
and
H.Eklund
(2005).
Structural basis for the changed substrate specificity of Drosophila melanogaster deoxyribonucleoside kinase mutant N64D.
|
| |
FEBS J,
272,
3733-3742.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Chakrabarti,
A.M.Klibanov,
and
R.A.Friesner
(2005).
Sequence optimization and designability of enzyme active sites.
|
| |
Proc Natl Acad Sci U S A,
102,
12035-12040.
|
 |
|
|
|
|
 |
H.Frederiksen,
D.Berenstein,
and
B.Munch-Petersen
(2004).
Effect of valine 106 on structure-function relation of cytosolic human thymidine kinase. Kinetic properties and oligomerization pattern of nine substitution mutants of V106.
|
| |
Eur J Biochem,
271,
2248-2256.
|
 |
|
|
|
|
 |
J.F.Barroso,
M.Elholm,
and
T.Flatmark
(2003).
Tight binding of deoxyribonucleotide triphosphates to human thymidine kinase 2 expressed in Escherichia coli. Purification and partial characterization of its dimeric and tetrameric forms.
|
| |
Biochemistry,
42,
15158-15169.
|
 |
|
|
|
|
 |
L.Salviati,
S.Sacconi,
M.Mancuso,
D.Otaegui,
P.Camaño,
A.Marina,
S.Rabinowitz,
R.Shiffman,
K.Thompson,
C.M.Wilson,
A.Feigenbaum,
A.B.Naini,
M.Hirano,
E.Bonilla,
S.DiMauro,
and
T.H.Vu
(2002).
Mitochondrial DNA depletion and dGK gene mutations.
|
| |
Ann Neurol,
52,
311-317.
|
 |
|
|
|
|
 |
M.Tramier,
I.Gautier,
T.Piolot,
S.Ravalet,
K.Kemnitz,
J.Coppey,
C.Durieux,
V.Mignotte,
and
M.Coppey-Moisan
(2002).
Picosecond-hetero-FRET microscopy to probe protein-protein interactions in live cells.
|
| |
Biophys J,
83,
3570-3577.
|
 |
|
|
|
|
 |
R.G.Zhang,
J.Grembecka,
E.Vinokour,
F.Collart,
I.Dementieva,
W.Minor,
and
A.Joachimiak
(2002).
Structure of Bacillus subtilis YXKO--a member of the UPF0031 family and a putative kinase.
|
| |
J Struct Biol,
139,
161-170.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Knecht,
M.P.Sandrini,
K.Johansson,
H.Eklund,
B.Munch-Petersen,
and
J.Piskur
(2002).
A few amino acid substitutions can convert deoxyribonucleoside kinase specificity from pyrimidines to purines.
|
| |
EMBO J,
21,
1873-1880.
|
 |
|
|
|
|
 |
C.Wurth,
U.Kessler,
J.Vogt,
G.E.Schulz,
G.Folkers,
and
L.Scapozza
(2001).
The effect of substrate binding on the conformation and structural stability of Herpes simplex virus type 1 thymidine kinase.
|
| |
Protein Sci,
10,
63-73.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.Gautier,
M.Tramier,
C.Durieux,
J.Coppey,
R.B.Pansu,
J.C.Nicolas,
K.Kemnitz,
and
M.Coppey-Moisan
(2001).
Homo-FRET microscopy in living cells to measure monomer-dimer transition of GFP-tagged proteins.
|
| |
Biophys J,
80,
3000-3008.
|
 |
|
|
|
|
 |
R.T.Sarisky,
R.Cano,
T.T.Nguyen,
R.J.Wittrock,
K.E.Duffy,
P.Clark,
J.O.Bartus,
T.H.Bacon,
L.Caspers-Velu,
R.L.Hodinka,
and
J.J.Leary
(2001).
Biochemical characterization of a virus isolate, recovered from a patient with herpes keratitis, that was clinically resistant to acyclovir.
|
| |
Clin Infect Dis,
33,
2034-2039.
|
 |
|
|
|
|
 |
A.Gorokhov,
L.Perera,
T.A.Darden,
M.Negishi,
L.C.Pedersen,
and
L.G.Pedersen
(2000).
Heparan sulfate biosynthesis: a theoretical study of the initial sulfation step by N-deacetylase/N-sulfotransferase.
|
| |
Biophys J,
79,
2909-2917.
|
 |
|
|
|
|
 |
A.Prota,
J.Vogt,
B.Pilger,
R.Perozzo,
C.Wurth,
V.E.Marquez,
P.Russ,
G.E.Schulz,
G.Folkers,
and
L.Scapozza
(2000).
Kinetics and crystal structure of the wild-type and the engineered Y101F mutant of Herpes simplex virus type 1 thymidine kinase interacting with (North)-methanocarba-thymidine.
|
| |
Biochemistry,
39,
9597-9603.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.J.MacRae,
I.H.Segel,
and
A.J.Fisher
(2000).
Crystal structure of adenosine 5'-phosphosulfate kinase from Penicillium chrysogenum.
|
| |
Biochemistry,
39,
1613-1621.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Vogt,
R.Perozzo,
A.Pautsch,
A.Prota,
P.Schelling,
B.Pilger,
G.Folkers,
L.Scapozza,
and
G.E.Schulz
(2000).
Nucleoside binding site of herpes simplex type 1 thymidine kinase analyzed by X-ray crystallography.
|
| |
Proteins,
41,
545-553.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Campobasso,
I.I.Mathews,
T.P.Begley,
and
S.E.Ealick
(2000).
Crystal structure of 4-methyl-5-beta-hydroxyethylthiazole kinase from Bacillus subtilis at 1.5 A resolution.
|
| |
Biochemistry,
39,
7868-7877.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Zheng,
and
J.S.Blanchard
(2000).
Identification of active site residues in E. coli ketopantoate reductase by mutagenesis and chemical rescue.
|
| |
Biochemistry,
39,
16244-16251.
|
 |
|
|
|
|
 |
T.A.Hinds,
C.Compadre,
B.K.Hurlburt,
and
R.R.Drake
(2000).
Conservative mutations of glutamine-125 in herpes simplex virus type 1 thymidine kinase result in a ganciclovir kinase with minimal deoxypyrimidine kinase activities.
|
| |
Biochemistry,
39,
4105-4111.
|
 |
|
|
|
|
 |
F.Morfin,
D.Thouvenot,
M.De Turenne-Tessier,
B.Lina,
M.Aymard,
and
T.Ooka
(1999).
Phenotypic and genetic characterization of thymidine kinase from clinical strains of varicella-zoster virus resistant to acyclovir.
|
| |
Antimicrob Agents Chemother,
43,
2412-2416.
|
 |
|
|
|
|
 |
J.Wang,
D.Choudhury,
J.Chattopadhyaya,
and
S.Eriksson
(1999).
Stereoisomeric selectivity of human deoxyribonucleoside kinases.
|
| |
Biochemistry,
38,
16993-16999.
|
 |
|
|
|
|
 |
D.H.Harrison,
J.A.Runquist,
A.Holub,
and
H.M.Miziorko
(1998).
The crystal structure of phosphoribulokinase from Rhodobacter sphaeroides reveals a fold similar to that of adenylate kinase.
|
| |
Biochemistry,
37,
5074-5085.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.I.Mathews,
M.D.Erion,
and
S.E.Ealick
(1998).
Structure of human adenosine kinase at 1.5 A resolution.
|
| |
Biochemistry,
37,
15607-15620.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.N.Champness,
M.S.Bennett,
F.Wien,
R.Visse,
W.C.Summers,
P.Herdewijn,
E.de Clerq,
T.Ostrowski,
R.L.Jarvest,
and
M.R.Sanderson
(1998).
Exploring the active site of herpes simplex virus type-1 thymidine kinase by X-ray crystallography of complexes with aciclovir and other ligands.
|
| |
Proteins,
32,
350-361.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.H.Chen,
W.J.Cook,
K.L.Grove,
and
D.M.Coen
(1998).
Human thymidine kinase can functionally replace herpes simplex virus type 1 thymidine kinase for viral replication in mouse sensory ganglia and reactivation from latency upon explant.
|
| |
J Virol,
72,
6710-6715.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

