 |
PDBsum entry 1e2m

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.1.21
- thymidine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
thymidine + ATP = dTMP + ADP + H+
|
 |
 |
 |
 |
 |
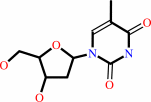 thymidine
thymidine
|
+
|
ATP
Bound ligand (Het Group name = )
matches with 76.47% similarity
|
=
|
 dTMP
dTMP
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Protein Sci
10:63-73
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
The effect of substrate binding on the conformation and structural stability of Herpes simplex virus type 1 thymidine kinase.
|
|
C.Wurth,
U.Kessler,
J.Vogt,
G.E.Schulz,
G.Folkers,
L.Scapozza.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structure of Herpes simplex virus type 1 thymidine kinase (TK(HSV1)) is
known at high resolution in complex with a series of ligands and exhibits
important structural similarities to the nucleoside monophosphate (NMP) kinase
family, which are known to show large conformational changes upon binding of
substrates. The effect of substrate binding on the conformation and structural
stability of TK(HSV1), measured by thermal denaturation experiments, far-UV
circular dichroism (CD) and fluorescence is described, and the results indicate
that the conformation of the ligand-free TK(HSV1) is less ordered and less
stable compared to the ligated enzyme. Furthermore, two crystal structures of
TK(HSV1) in complex with two new ligands, HPT and HMTT, refined to 2.2 A are
presented. Although TK(HSV1):HPT does not exhibit any significant deviations
from the model of TK(HSV1):dT, the TK(HSV1):HMTT complex displays a unique
conformationally altered active site resulting in a lowered thermal stability of
this complex. Moreover, we show that binding affinity and binding mode of the
ligand correlate with thermal stability of the complex. We use this correlation
to propose a method to estimate binding constants for new TK(HSV1)substrates
using thermal denaturation measurements monitored by CD spectroscopy. The
kinetic and structural results of both test substrates HPT and HMTT show that
the CD thermal denaturation system is very sensitive to conformational changes
caused by unusual binding of a substrate analog.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Caillat,
D.Topalis,
L.A.Agrofoglio,
S.Pochet,
J.Balzarini,
D.Deville-Bonne,
and
P.Meyer
(2008).
Crystal structure of poxvirus thymidylate kinase: an unexpected dimerization has implications for antiviral therapy.
|
| |
Proc Natl Acad Sci U S A,
105,
16900-16905.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Ramensky,
A.Sobol,
N.Zaitseva,
A.Rubinov,
and
V.Zosimov
(2007).
A novel approach to local similarity of protein binding sites substantially improves computational drug design results.
|
| |
Proteins,
69,
349-357.
|
 |
|
|
|
|
 |
P.Kapková,
E.Heller,
E.Kugelmann,
J.Faber,
G.Bringmann,
U.Kessler,
G.Folkers,
and
U.Holzgrabe
(2006).
Random chemistry as a new tool for the generation of small-compound libraries.
|
| |
Arch Pharm (Weinheim),
339,
489-497.
|
 |
|
|
|
|
 |
E.Kellenberger,
J.Rodrigo,
P.Muller,
and
D.Rognan
(2004).
Comparative evaluation of eight docking tools for docking and virtual screening accuracy.
|
| |
Proteins,
57,
225-242.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

