 |
PDBsum entry 3v98

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
3v98
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
S663d stable-5-lox
|
|
Structure:
|
 |
Arachidonate 5-lipoxygenase. Chain: a, b. Synonym: 5-lo, 5-lipoxygenase. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: alox5, log5. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.07Å
|
R-factor:
|
0.167
|
R-free:
|
0.197
|
|
|
Authors:
|
 |
N.C.Gilbert,Z.Rui,D.B.Neau,M.Waight,S.G.Bartlett,W.E.Boeglin, A.R.Brash,M.E.Newcomer
|
|
Key ref:
|
 |
N.C.Gilbert
et al.
(2012).
Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663.
Faseb J,
26,
3222-3229.
PubMed id:
|
 |
|
Date:
|
 |
|
23-Dec-11
|
Release date:
|
02-May-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P09917
(LOX5_HUMAN) -
Polyunsaturated fatty acid 5-lipoxygenase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
674 a.a.
669 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 12 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.13.11.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.1.13.11.34
- arachidonate 5-lipoxygenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate + O2 = leukotriene A4 + H2O
|
 |
 |
 |
 |
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate
|
+
|
 O2
O2
|
=
|
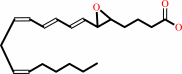 leukotriene A4
leukotriene A4
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe cation
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Faseb J
26:3222-3229
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663.
|
|
N.C.Gilbert,
Z.Rui,
D.B.Neau,
M.T.Waight,
S.G.Bartlett,
W.E.Boeglin,
A.R.Brash,
M.E.Newcomer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

