 |
PDBsum entry 3h0s

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.6.3.4.14
- biotin carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N6-biotinyl-L-lysyl-[protein] + hydrogencarbonate + ATP = N6- carboxybiotinyl-L-lysyl-[protein] + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
N(6)-biotinyl-L-lysyl-[protein]
|
+
|
 hydrogencarbonate
hydrogencarbonate
|
+
|
 ATP
ATP
|
=
|
N(6)- carboxybiotinyl-L-lysyl-[protein]
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.6.4.1.2
- acetyl-CoA carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydrogencarbonate + acetyl-CoA + ATP = malonyl-CoA + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 hydrogencarbonate
hydrogencarbonate
|
+
|
 acetyl-CoA
acetyl-CoA
|
+
|
 ATP
ATP
|
=
|
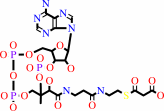 malonyl-CoA
malonyl-CoA
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Biotin
|
 |
 |
 |
 |
 |
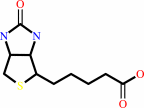 Biotin
Biotin
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Bioorg Med Chem Lett
20:2383-2388
(2010)
|
|
PubMed id:
|
|
|
|

|
| |
|
Discovery of small molecule isozyme non-specific inhibitors of mammalian acetyl-CoA carboxylase 1 and 2.
|
|
J.W.Corbett,
K.D.Freeman-Cook,
R.Elliott,
F.Vajdos,
F.Rajamohan,
D.Kohls,
E.Marr,
H.Zhang,
L.Tong,
M.Tu,
S.Murdande,
S.D.Doran,
J.A.Houser,
W.Song,
C.J.Jones,
S.B.Coffey,
L.Buzon,
M.L.Minich,
K.J.Dirico,
S.Tapley,
R.K.McPherson,
E.Sugarman,
H.J.Harwood,
W.Esler.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Screening Pfizer's compound library resulted in the identification of weak
acetyl-CoA carboxylase inhibitors, from which were obtained rACC1 CT-domain
co-crystal structures. Utilizing HTS hits and structure-based drug discovery, a
more rigid inhibitor was designed and led to the discovery of sub-micromolar,
spirochromanone non-specific ACC inhibitors. Low nanomolar, non-specific
ACC-isozyme inhibitors that exhibited good rat pharmacokinetics were obtained
from this chemotype.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Bengtsson,
S.Blaho,
D.B.Saitton,
K.Brickmann,
J.Broddefalk,
O.Davidsson,
T.Drmota,
R.Folmer,
K.Hallberg,
S.Hallén,
R.Hovland,
E.Isin,
P.Johannesson,
B.Kull,
L.O.Larsson,
L.Löfgren,
K.E.Nilsson,
T.Noeske,
N.Oakes,
A.T.Plowright,
V.Schnecke,
P.Ståhlberg,
P.Sörme,
H.Wan,
E.Wellner,
and
L.Oster
(2011).
Design of small molecule inhibitors of acetyl-CoA carboxylase 1 and 2 showing reduction of hepatic malonyl-CoA levels in vivo in obese Zucker rats.
|
| |
Bioorg Med Chem,
19,
3039-3053.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Marjanovic,
D.Chalupska,
C.Patenode,
A.Coster,
E.Arnold,
A.Ye,
G.Anesi,
Y.Lu,
I.Okun,
S.Tkachenko,
R.Haselkorn,
and
P.Gornicki
(2010).
Recombinant yeast screen for new inhibitors of human acetyl-CoA carboxylase 2 identifies potential drugs to treat obesity.
|
| |
Proc Natl Acad Sci U S A,
107,
9093-9098.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

