 |
PDBsum entry 3cmd

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.1.88
- peptide deformylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-terminal N-formyl-L-methionyl-[peptide] + H2O = N-terminal L-methionyl- [peptide] + formate
|
 |
 |
 |
 |
 |
N-terminal N-formyl-L-methionyl-[peptide]
|
+
|
H2O
|
=
|
N-terminal L-methionyl- [peptide]
Bound ligand (Het Group name = )
matches with 42.86% similarity
|
+
|
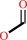 formate
formate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proteins
74:261-265
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Insight into the antibacterial drug design and architectural mechanism of peptide recognition from the E. faecium peptide deformylase structure.
|
|
K.H.Nam,
J.I.Ham,
A.Priyadarshi,
E.E.Kim,
N.Chung,
K.Y.Hwang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Crystal structure of EfPDF. (a) Crystallographic
packing of 75.4% solvent content. Twelve of the EfPDF monomers
generated a large 80 Å pore. (b) Unfolded N-terminal
expression tag peptides of all molecules accessed the active
site pockets of neighboring molecules. The expression peptide
has  82%
buried surfaced area, but makes no contacts. A 2Fo-Fc electron
density map contoured at 1 82%
buried surfaced area, but makes no contacts. A 2Fo-Fc electron
density map contoured at 1  is
shown in gray in the N-terminal region of the molecule.
Molecules A and B are represented by green and cyan,
respectively. The expression tag region is represented by
dark-blue sticks. The surface of the active site pocket is
represented in red. (c) Ribbon diagram of the EfPDF monomer
structure. The unfolded expression tag peptide in the N-terminal
region lies along the disordered region located between is
shown in gray in the N-terminal region of the molecule.
Molecules A and B are represented by green and cyan,
respectively. The expression tag region is represented by
dark-blue sticks. The surface of the active site pocket is
represented in red. (c) Ribbon diagram of the EfPDF monomer
structure. The unfolded expression tag peptide in the N-terminal
region lies along the disordered region located between  2
and 2
and  3
(CD loop). Helices are represented in red, 3
(CD loop). Helices are represented in red,  strands
in yellow, loops in green and the expression tag peptide loop in
light blue. The active site metal ion is represented by the
orange sphere. (d) Malonic acid interacts with the metal ion and
Glu158, which is the catalytic residue. The active site pocket
is loosely associated with the malonic acid. The active site
residues are shown by ball-and-stick representations. The metal
ion is represented by the orange sphere. strands
in yellow, loops in green and the expression tag peptide loop in
light blue. The active site metal ion is represented by the
orange sphere. (d) Malonic acid interacts with the metal ion and
Glu158, which is the catalytic residue. The active site pocket
is loosely associated with the malonic acid. The active site
residues are shown by ball-and-stick representations. The metal
ion is represented by the orange sphere.
|
 |
Figure 2.
Figure 2. Proposed architectural mechanism of peptide
recognition. Approximately 15 × 10 Å  U U
 shaped
cavity in the EfPDF crystal structure. This shape prevents
access by folded structures containing shaped
cavity in the EfPDF crystal structure. This shape prevents
access by folded structures containing  -helices
or -helices
or  -sheets.
Unfolded peptides can access the site from the side. The black
dotted circle shows the region which prevents structurally
folded proteins from entering, thus increasing the selectivity
for unfolded peptides. The CD loop is located in the area of the
red dotted circle. -sheets.
Unfolded peptides can access the site from the side. The black
dotted circle shows the region which prevents structurally
folded proteins from entering, thus increasing the selectivity
for unfolded peptides. The CD loop is located in the area of the
red dotted circle.  X X
 indicates
a route of entry that is likely to be inaccessible. Green ribbon
is unfolded N-terminal expression tag peptides in our result. indicates
a route of entry that is likely to be inaccessible. Green ribbon
is unfolded N-terminal expression tag peptides in our result.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from John Wiley & Sons, Inc.:
Proteins
(2009,
74,
261-265)
copyright 2009.
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

