
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D:
E.C.1.15.1.1
- superoxide dismutase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 superoxide + 2 H+ = H2O2 + O2
|
 |
 |
 |
 |
 |
2
×
superoxide
|
+
|
2
×
H(+)
|
=
|
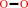 H2O2
H2O2
|
+
|
 O2
O2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe cation or Mn(2+) or (Zn(2+) and Cu cation)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
365:333-342
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
The coupling between disulphide status, metallation and dimer interface strength in Cu/Zn superoxide dismutase.
|
|
A.Hörnberg,
D.T.Logan,
S.L.Marklund,
M.Oliveberg.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The gain of neurotoxic function in amyotrophic lateral sclerosis (ALS) has been
linked to misfolding of the homodimeric enzyme Cu/Zn superoxide dismutase (SOD).
Here, we present the crystal structure of fully cysteine-depleted human SOD
(SOD(CallA)), representing a reduced, marginally stable intermediate on the
folding pathway in vivo that has also been implicated as neurotoxic precursor
state. A hallmark of this species is that it fails to dimerize and becomes
trapped as a monomer in the absence of the active-site metals. The
crystallographic data show that removal of the C57-C146 disulphide bond sets
free the interface loop IV in the apo protein, whereas the same loop remains
unaffected in the holo protein. Thus, the low dimerisation propensity of
disulphide-reduced apoSOD seems to be of entropic origin due to increased loop
flexibility in the monomeric state: in the disulphide-reduced holo protein this
gain in configurational entropy upon splitting of the dimer interface is reduced
by the metal coordination.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Changes of the homodimer interface of the fully
cysteine-depleted variant apoSOD^CallA. (a) The apoSOD^CallA
dimer. (b) The interface areas of the individual apoSOD^CallA
monomers indicating the contacts that are lost, weakened and
maintained upon truncation of the C57–C146 disulphide link and
movement of loop IV. Figure 3. Changes of the homodimer
interface of the fully cysteine-depleted variant apoSOD^CallA.
(a) The apoSOD^CallA dimer. (b) The interface areas of the
individual apoSOD^CallA monomers indicating the contacts that
are lost, weakened and maintained upon truncation of the
C57–C146 disulphide link and movement of loop IV.
|
 |
Figure 4.
Figure 4. Displacement of Arg143 in apoSOD^CallA provides a
clue to the structural origin of decreased activity in monomeric
and disulphide-reduced protein. Accompanying the structural
alteration of loop IV, the catalytically important guanidinium
group of R143 moves from its native position and forms new
hydrogen bonds with S59, D52 and the water molecules Wat26 and
Wat68. (a) The 2F[o]–F[c] electron density map of apoSOD^CallA
at 1σ. (b) Schematic representation of the apoSOD^CallA
structure illustrating the hydrogen bonding to R143. (c)
Comparison of the loop IV conformations and positioning of R143
by superposition of the A monomers of apoSOD^CallA (blue) and
holoSOD^CallA (red). Figure 4. Displacement of Arg143 in
apoSOD^CallA provides a clue to the structural origin of
decreased activity in monomeric and disulphide-reduced protein.
Accompanying the structural alteration of loop IV, the
catalytically important guanidinium group of R143 moves from its
native position and forms new hydrogen bonds with S59, D52 and
the water molecules Wat26 and Wat68. (a) The 2F[o]–F[c]
electron density map of apoSOD^CallA at 1σ. (b) Schematic
representation of the apoSOD^CallA structure illustrating the
hydrogen bonding to R143. (c) Comparison of the loop IV
conformations and positioning of R143 by superposition of the A
monomers of apoSOD^CallA (blue) and holoSOD^CallA (red).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2007,
365,
333-342)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.A.Proctor,
F.Ding,
and
N.V.Dokholyan
(2011).
Structural and thermodynamic effects of post-translational modifications in mutant and wild type Cu, Zn superoxide dismutase.
|
| |
J Mol Biol,
408,
555-567.
|
 |
|
|
|
|
 |
L.R.Fischer,
A.Igoudjil,
J.Magrané,
Y.Li,
J.M.Hansen,
G.Manfredi,
and
J.D.Glass
(2011).
SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse.
|
| |
Brain,
134,
196-209.
|
 |
|
|
|
|
 |
A.K.Svensson,
O.Bilsel,
C.Kayatekin,
J.A.Adefusika,
J.A.Zitzewitz,
and
C.R.Matthews
(2010).
Metal-free ALS variants of dimeric human Cu,Zn-superoxide dismutase have enhanced populations of monomeric species.
|
| |
PLoS One,
5,
e10064.
|
 |
|
|
|
|
 |
J.R.Auclair,
K.J.Boggio,
G.A.Petsko,
D.Ringe,
and
J.N.Agar
(2010).
Strategies for stabilizing superoxide dismutase (SOD1), the protein destabilized in the most common form of familial amyotrophic lateral sclerosis.
|
| |
Proc Natl Acad Sci U S A,
107,
21394-21399.
|
 |
|
|
|
|
 |
A.Nordlund,
L.Leinartaite,
K.Saraboji,
C.Aisenbrey,
G.Gröbner,
P.Zetterström,
J.Danielsson,
D.T.Logan,
and
M.Oliveberg
(2009).
Functional features cause misfolding of the ALS-provoking enzyme SOD1.
|
| |
Proc Natl Acad Sci U S A,
106,
9667-9672.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Tiwari,
A.Liba,
S.H.Sohn,
S.V.Seetharaman,
O.Bilsel,
C.R.Matthews,
P.J.Hart,
J.S.Valentine,
and
L.J.Hayward
(2009).
Metal deficiency increases aberrant hydrophobicity of mutant superoxide dismutases that cause amyotrophic lateral sclerosis.
|
| |
J Biol Chem,
284,
27746-27758.
|
 |
|
|
|
|
 |
J.M.Leitch,
L.T.Jensen,
S.D.Bouldin,
C.E.Outten,
P.J.Hart,
and
V.C.Culotta
(2009).
Activation of Cu,Zn-superoxide dismutase in the absence of oxygen and the copper chaperone CCS.
|
| |
J Biol Chem,
284,
21863-21871.
|
 |
|
|
|
|
 |
K.A.Trumbull,
and
J.S.Beckman
(2009).
A role for copper in the toxicity of zinc-deficient superoxide dismutase to motor neurons in amyotrophic lateral sclerosis.
|
| |
Antioxid Redox Signal,
11,
1627-1639.
|
 |
|
|
|
|
 |
A.Nordlund,
and
M.Oliveberg
(2008).
SOD1-associated ALS: a promising system for elucidating the origin of protein-misfolding disease.
|
| |
HFSP J,
2,
354-364.
|
 |
|
|
|
|
 |
C.Kayatekin,
J.A.Zitzewitz,
and
C.R.Matthews
(2008).
Zinc binding modulates the entire folding free energy surface of human Cu,Zn superoxide dismutase.
|
| |
J Mol Biol,
384,
540-555.
|
 |
|
|
|
|
 |
F.Ding,
and
N.V.Dokholyan
(2008).
Dynamical roles of metal ions and the disulfide bond in Cu, Zn superoxide dismutase folding and aggregation.
|
| |
Proc Natl Acad Sci U S A,
105,
19696-19701.
|
 |
|
|
|
|
 |
H.Kawamata,
and
G.Manfredi
(2008).
Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria.
|
| |
Hum Mol Genet,
17,
3303-3317.
|
 |
|
|
|
|
 |
B.R.Roberts,
J.A.Tainer,
E.D.Getzoff,
D.A.Malencik,
S.R.Anderson,
V.C.Bomben,
K.R.Meyers,
P.A.Karplus,
and
J.S.Beckman
(2007).
Structural characterization of zinc-deficient human superoxide dismutase and implications for ALS.
|
| |
J Mol Biol,
373,
877-890.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

