 |
PDBsum entry 2d2h

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.8.1
- aryldialkylphosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
An aryl dialkyl phosphate + H2O = dialkyl phosphate + an aryl alcohol
|
 |
 |
 |
 |
 |
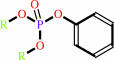 aryl dialkyl phosphate
aryl dialkyl phosphate
|
+
|
H2O
|
=
|
dialkyl phosphate
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
+
|
aryl alcohol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Divalent cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochim Biophys Acta
1752:56-64
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of an enzyme-product complex reveals the critical role of a terminal hydroxide nucleophile in the bacterial phosphotriesterase mechanism.
|
|
C.Jackson,
H.K.Kim,
P.D.Carr,
J.W.Liu,
D.L.Ollis.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A detailed understanding of the catalytic mechanism of enzymes is an important
step toward improving their activity for use in biotechnology. In this paper,
crystal soaking experiments and X-ray crystallography were used to analyse the
mechanism of the Agrobacterium radiobacter phosphotriesterase, OpdA, an enzyme
capable of detoxifying a broad range of organophosphate pesticides. The
structures of OpdA complexed with ethylene glycol and the product of dimethoate
hydrolysis, dimethyl thiophosphate, provide new details of the catalytic
mechanism. These structures suggest that the attacking nucleophile is a
terminally bound hydroxide, consistent with the catalytic mechanism of other
binuclear metallophosphoesterases. In addition, a crystal structure with the
potential substrate trimethyl phosphate bound non-productively demonstrates the
importance of the active site cavity in orienting the substrate into an
approximation of the transition state.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.S.Fraser,
and
C.J.Jackson
(2011).
Mining electron density for functionally relevant protein polysterism in crystal structures.
|
| |
Cell Mol Life Sci,
68,
1829-1841.
|
 |
|
|
|
|
 |
J.A.Larrabee,
W.R.Johnson,
and
A.S.Volwiler
(2009).
Magnetic circular dichroism study of a dicobalt(II) complex with mixed 5- and 6-coordination: a spectroscopic model for dicobalt(II) hydrolases.
|
| |
Inorg Chem,
48,
8822-8829.
|
 |
|
|
|
|
 |
P.Del Vecchio,
M.Elias,
L.Merone,
G.Graziano,
J.Dupuy,
L.Mandrich,
P.Carullo,
B.Fournier,
D.Rochu,
M.Rossi,
P.Masson,
E.Chabriere,
and
G.Manco
(2009).
Structural determinants of the high thermal stability of SsoPox from the hyperthermophilic archaeon Sulfolobus solfataricus.
|
| |
Extremophiles,
13,
461-470.
|
 |
|
|
|
|
 |
X.Zhang,
R.Wu,
L.Song,
Y.Lin,
M.Lin,
Z.Cao,
W.Wu,
and
Y.Mo
(2009).
Molecular dynamics simulations of the detoxification of paraoxon catalyzed by phosphotriesterase.
|
| |
J Comput Chem,
30,
2388-2401.
|
 |
|
|
|
|
 |
C.J.Jackson,
K.S.Hadler,
P.D.Carr,
A.J.Oakley,
S.Yip,
G.Schenk,
and
D.L.Ollis
(2008).
Malonate-bound structure of the glycerophosphodiesterase from Enterobacter aerogenes (GpdQ) and characterization of the native Fe2+ metal-ion preference.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
681-685.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Kim,
P.C.Tsai,
S.L.Chen,
F.Himo,
S.C.Almo,
and
F.M.Raushel
(2008).
Structure of diethyl phosphate bound to the binuclear metal center of phosphotriesterase.
|
| |
Biochemistry,
47,
9497-9504.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.E.Mirams,
S.J.Smith,
K.S.Hadler,
D.L.Ollis,
G.Schenk,
and
L.R.Gahan
(2008).
Cadmium(II) complexes of the glycerophosphodiester-degrading enzyme GpdQ and a biomimetic N,O ligand.
|
| |
J Biol Inorg Chem,
13,
1065-1072.
|
 |
|
|
|
|
 |
D.E.Danley
(2006).
Crystallization to obtain protein-ligand complexes for structure-aided drug design.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
569-575.
|
 |
|
|
|
|
 |
C.J.Jackson,
J.W.Liu,
M.L.Coote,
and
D.L.Ollis
(2005).
The effects of substrate orientation on the mechanism of a phosphotriesterase.
|
| |
Org Biomol Chem,
3,
4343-4350.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

