 |
PDBsum entry 2c64

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2c64
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Mao inhibition by rasagiline analogues
|
|
Structure:
|
 |
Amine oxidase (flavin-containing) b. Chain: a, b. Synonym: monoamine oxidase b, mao-b. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Expressed in: pichia pastoris. Expression_system_taxid: 4922
|
|
Biol. unit:
|
 |
Dimer (from PDB file)
|
|
Resolution:
|
 |
|
2.20Å
|
R-factor:
|
0.223
|
R-free:
|
0.260
|
|
|
Authors:
|
 |
C.Binda,F.Hubalek,M.Li,Y.Herzig,J.Sterling,D.E.Edmondson,A.Mattevi
|
|
Key ref:
|
 |
C.Binda
et al.
(2005).
Binding of rasagiline-related inhibitors to human monoamine oxidases: a kinetic and crystallographic analysis.
J Med Chem,
48,
8148-8154.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
07-Nov-05
|
Release date:
|
04-Jan-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P27338
(AOFB_HUMAN) -
Amine oxidase [flavin-containing] B from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
520 a.a.
499 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.4.3.21
- primary-amine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a primary methyl amine + O2 + H2O = an aldehyde + H2O2 + NH4+
|
 |
 |
 |
 |
 |
primary methyl amine
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
 aldehyde
aldehyde
|
+
|
 H2O2
H2O2
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.1.4.3.4
- monoamine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a secondary aliphatic amine + O2 + H2O = a primary amine + an aldehyde + H2O2
|
 |
 |
 |
 |
 |
secondary aliphatic amine
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
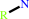 primary amine
primary amine
|
+
|
 aldehyde
aldehyde
|
+
|
 H2O2
H2O2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
48:8148-8154
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Binding of rasagiline-related inhibitors to human monoamine oxidases: a kinetic and crystallographic analysis.
|
|
C.Binda,
F.Hubálek,
M.Li,
Y.Herzig,
J.Sterling,
D.E.Edmondson,
A.Mattevi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Monoamine oxidases A and B (MAO A and B) catalyze the degradation of
neurotransmitters and represent drug targets for the treatment of
neurodegenerative disorders. Rasagiline is an irreversible, MAO B-selective
inhibitor that has been approved as a novel anti-Parkinson's drug. In this
study, we investigate the inhibition of recombinant human MAO A and MAO B by
several rasagiline analogues. Different substituents added onto the rasagiline
scaffold alter the binding affinity depending on the position on the aminoindan
ring and on the size of the substituent. Compounds with a hydroxyl group on
either the C4 or the C6 atom inhibit both isozymes, whereas a bulkier
substituent such as a carbamate is tolerated only at the C4 position. The 1.7 A
crystal structure of MAO B in complex with
4-(N-methyl-N-ethyl-carbamoyloxy)-N-methyl-N-propargyl-1(R)-aminoindan shows
that the binding mode is similar to that of rasagiline with the carbamate moiety
occupying the entrance cavity space. 1(R)-Aminoindan, the major metabolic
product of rasagiline, and its analogues reversibly inhibit both MAO A and MAO
B. The crystal structure of N-methyl-1(R)-aminoindan bound to MAO B shows that
its aminoindan ring adopts a different orientation compared to that of
rasagiline.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
O.Weinreb,
O.Bar-Am,
K.Prosolovich,
T.Amit,
and
M.B.Youdim
(2011).
Does 1-(R)-aminoindan Possess Neuroprotective Properties Against Experimental Parkinson's Disease?
|
| |
Antioxid Redox Signal,
14,
767-775.
|
 |
|
|
|
|
 |
O.Bar-Am,
O.Weinreb,
T.Amit,
and
M.B.Youdim
(2010).
The neuroprotective mechanism of 1-(R)-aminoindan, the major metabolite of the anti-parkinsonian drug rasagiline.
|
| |
J Neurochem,
112,
1131-1137.
|
 |
|
|
|
|
 |
O.Weinreb,
T.Amit,
O.Bar-Am,
and
M.B.Youdim
(2010).
Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity.
|
| |
Prog Neurobiol,
92,
330-344.
|
 |
|
|
|
|
 |
Z.Jia,
S.Wei,
and
Q.Zhu
(2010).
Monoamine oxidase inhibitors: benzylidene-prop-2-ynyl-amines analogues.
|
| |
Biol Pharm Bull,
33,
725-728.
|
 |
|
|
|
|
 |
D.E.Edmondson,
C.Binda,
J.Wang,
A.K.Upadhyay,
and
A.Mattevi
(2009).
Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases.
|
| |
Biochemistry,
48,
4220-4230.
|
 |
|
|
|
|
 |
M.Naoi,
and
W.Maruyama
(2009).
Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson's disease.
|
| |
Expert Rev Neurother,
9,
1233-1250.
|
 |
|
|
|
|
 |
D.B.Langley,
D.M.Trambaiolo,
A.P.Duff,
D.M.Dooley,
H.C.Freeman,
and
J.M.Guss
(2008).
Complexes of the copper-containing amine oxidase from Arthrobacter globiformis with the inhibitors benzylhydrazine and tranylcypromine.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
577-583.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.W.van Hellemond,
M.van Dijk,
D.P.Heuts,
D.B.Janssen,
and
M.W.Fraaije
(2008).
Discovery and characterization of a putrescine oxidase from Rhodococcus erythropolis NCIMB 11540.
|
| |
Appl Microbiol Biotechnol,
78,
455-463.
|
 |
|
|
|
|
 |
T.A.White,
W.H.Johnson,
C.P.Whitman,
and
J.J.Tanner
(2008).
Structural basis for the inactivation of Thermus thermophilus proline dehydrogenase by N-propargylglycine.
|
| |
Biochemistry,
47,
5573-5580.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Fierro,
M.Osorio-Olivares,
B.K.Cassels,
D.E.Edmondson,
S.Sepúlveda-Boza,
and
M.Reyes-Parada
(2007).
Human and rat monoamine oxidase-A are differentially inhibited by (S)-4-alkylthioamphetamine derivatives: insights from molecular modeling studies.
|
| |
Bioorg Med Chem,
15,
5198-5206.
|
 |
|
|
|
|
 |
L.M.Szewczuk,
J.C.Culhane,
M.Yang,
A.Majumdar,
H.Yu,
and
P.A.Cole
(2007).
Mechanistic analysis of a suicide inactivator of histone demethylase LSD1.
|
| |
Biochemistry,
46,
6892-6902.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

