 |
PDBsum entry 2bpp

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Carboxylic ester hydrolase
|
PDB id
|
|

|

|
2bpp
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
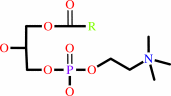 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
30:11801-11811
(1991)
|
|
PubMed id:
|
|
|
|

|
| |
|
Phospholipase A2 engineering. X-ray structural and functional evidence for the interaction of lysine-56 with substrates.
|
|
J.P.Noel,
C.A.Bingman,
T.L.Deng,
C.M.Dupureur,
K.J.Hamilton,
R.T.Jiang,
J.G.Kwak,
C.Sekharudu,
M.Sundaralingam,
M.D.Tsai.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Site-directed mutagenesis studies of bovine pancreatic phospholipase A2 (PLA2,
overproduced in Escherichia coli) showed that replacement of surface residue
Lys-56 by a neutral or hydrophobic amino acid residue resulted in an unexpected
and significant change in the function of the enzyme. The kcat for
phosphatidylcholine micelles increases 3-4-fold for K56M, K56I, and K56F and ca.
2-fold for K56N and K56T but does not change for K56R. These results suggest
that the side chain of residue 56 has significant influence on the activity of
PLA2. In order to probe the structural basis for the enhanced activity, the
crystal structures of wild-type and K56M PLA2 were determined by X-ray
crystallography to a resolution of 1.8 A. The results suggest that the mutation
has not only perturbed the conformation of the side chain of Met-56 locally but
also caused conformational changes in the neighboring loop (residues 60-70),
resulting in the formation of a hydrophobic pocket by residues Met-56, Tyr-52,
and Tyr-69. Docking of a phosphatidylcholine inhibitor analogue into the active
site of K56M, according to the structure of the complex of cobra venom
PLA2-phosphatidylethanolamine inhibitor analogue [White, S.P., Scott, D. L.,
Otwinowski, Z., Gleb, M. H., & Sigler, P. (1990) Science 250, 1560-1563],
showed that the choline moiety [N(CH3)3]+ is readily accommodated into the newly
formed hydrophobic pocket with a high degree of surface complementarity. This
suggests a possible interaction between residue 56 and the head group of the
phospholipid, explaining the enhanced activities observed when the positively
charged Lys-56 is substituted by apolar residues, viz., K56M, K56I, and K56F.
Further support for this interpretation comes from the 5-fold enhancement in
kcat for the mutant K56E with a negatively charged side chain, where there would
be an attractive electrostatic interaction between the side chain of Glu-56 and
the positively charged choline moiety. Our results also refute a recent report
[Tomasselli, A. G., Hui, J., Fisher, J., Zürcher-Neely, H., Reardon, I.M.,
Oriaku, E., Kézdy, F.J., & Heinrikson, R.L. (1989) J. Biol. Chem. 264,
10041-10047] that substrate-level acylation of Lys-56 is an obligatory step in
the catalysis by PLA2.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Tanaka,
K.Fukase,
and
S.Katsumura
(2010).
New strategy in synthetic biology: from enzyme inhibition and natural products synthesis to PET imaging by 6pi-azaelectrocyclization.
|
| |
Chem Rec,
10,
119-139.
|
 |
|
|
|
|
 |
V.D.Mouchlis,
T.M.Mavromoustakos,
and
G.Kokotos
(2010).
Design of new secreted phospholipase A2 inhibitors based on docking calculations by modifying the pharmacophore segments of the FPL67047XX inhibitor.
|
| |
J Comput Aided Mol Des,
24,
107-115.
|
 |
|
|
|
|
 |
S.P.Kanaujia,
and
K.Sekar
(2008).
Structures and molecular-dynamics studies of three active-site mutants of bovine pancreatic phospholipase A(2).
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
1003-1011.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.De Maria,
J.Vind,
K.M.Oxenbøll,
A.Svendsen,
and
S.Patkar
(2007).
Phospholipases and their industrial applications.
|
| |
Appl Microbiol Biotechnol,
74,
290-300.
|
 |
|
|
|
|
 |
K.Sekar,
M.Yogavel,
D.Gayathri,
D.Velmurugan,
R.Krishna,
M.J.Poi,
Z.Dauter,
M.Dauter,
and
M.D.Tsai
(2006).
Atomic resolution structure of the double mutant (K53,56M) of bovine pancreatic phospholipase A2.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
1-5.
|
 |
|
|
|
|
 |
K.Sekar,
V.Rajakannan,
D.Velmurugan,
T.Yamane,
R.Thirumurugan,
M.Dauter,
and
Z.Dauter
(2004).
A redetermination of the structure of the triple mutant (K53,56,120M) of phospholipase A2 at 1.6 A resolution using sulfur-SAS at 1.54 A wavelength.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1586-1590.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.Zhao,
H.Liao,
and
M.D.Tsai
(2004).
The catalytic role of aspartate in a short strong hydrogen bond of the Asp274-His32 catalytic dyad in phosphatidylinositol-specific phospholipase C can be substituted by a chloride ion.
|
| |
J Biol Chem,
279,
31995-32000.
|
 |
|
|
|
|
 |
H.J.Kim,
and
M.P.Foster
(2002).
Characterization of Ad5 E3-14.7K, an adenoviral inhibitor of apoptosis: structure, oligomeric state, and metal binding.
|
| |
Protein Sci,
11,
1117-1128.
|
 |
|
|
|
|
 |
Y.Matoba,
Y.Katsube,
and
M.Sugiyama
(2002).
The crystal structure of prokaryotic phospholipase A2.
|
| |
J Biol Chem,
277,
20059-20069.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.Z.Yu,
M.J.Poi,
U.A.Ramagopal,
R.Jain,
S.Ramakumar,
O.G.Berg,
M.D.Tsai,
K.Sekar,
and
M.K.Jain
(2000).
Structural basis of the anionic interface preference and kcat* activation of pancreatic phospholipase A2.
|
| |
Biochemistry,
39,
12312-12323.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.Iijima,
S.Uchiyama,
Y.Fujikawa,
and
M.Esaka
(2000).
Purification, characterization, and molecular cloning of group I phospholipases A2 from the gills of the red sea bream, Pagrus major.
|
| |
Lipids,
35,
1359-1370.
|
 |
|
|
|
|
 |
B.Z.Yu,
J.Rogers,
M.D.Tsai,
C.Pidgeon,
and
M.K.Jain
(1999).
Contributions of residues of pancreatic phospholipase A2 to interfacial binding, catalysis, and activation.
|
| |
Biochemistry,
38,
4875-4884.
|
 |
|
|
|
|
 |
C.Yuan,
I.J.Byeon,
Y.Li,
and
M.D.Tsai
(1999).
Structural analysis of phospholipase A2 from functional perspective. 1. Functionally relevant solution structure and roles of the hydrogen-bonding network.
|
| |
Biochemistry,
38,
2909-2918.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Yuan,
and
M.Tsai
(1999).
Pancreatic phospholipase A(2): new views on old issues.
|
| |
Biochim Biophys Acta,
1441,
215-222.
|
 |
|
|
|
|
 |
K.Sekar,
and
M.Sundaralingam
(1999).
High-resolution refinement of orthorhombic bovine pancreatic phospholipase A2.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
46-50.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.J.Janssen,
P.J.Burghout,
H.M.Verheij,
A.J.Slotboom,
and
M.R.Egmond
(1999).
Introduction of a C-terminal aromatic sequence from snake venom phospholipases A2 into the porcine pancreatic isozyme dramatically changes the interfacial kinetics.
|
| |
Eur J Biochem,
263,
782-788.
|
 |
|
|
|
|
 |
J.Rogers,
B.Z.Yu,
M.D.Tsai,
O.G.Berg,
and
M.K.Jain
(1998).
Cationic residues 53 and 56 control the anion-induced interfacial k*cat activation of pancreatic phospholipase A2.
|
| |
Biochemistry,
37,
9549-9556.
|
 |
|
|
|
|
 |
K.Sekar,
C.Sekharudu,
M.D.Tsai,
and
M.Sundaralingam
(1998).
1.72 A resolution refinement of the trigonal form of bovine pancreatic phospholipase A2.
|
| |
Acta Crystallogr D Biol Crystallogr,
54,
342-346.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Sekar,
B.Z.Yu,
J.Rogers,
J.Lutton,
X.Liu,
X.Chen,
M.D.Tsai,
M.K.Jain,
and
M.Sundaralingam
(1997).
Phospholipase A2 engineering. Structural and functional roles of the highly conserved active site residue aspartate-99.
|
| |
Biochemistry,
36,
3104-3114.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.Huang,
B.Z.Yu,
J.Rogers,
I.J.Byeon,
K.Sekar,
X.Chen,
M.Sundaralingam,
M.D.Tsai,
and
M.K.Jain
(1996).
Phospholipase A2 engineering. Deletion of the C-terminus segment changes substrate specificity and uncouples calcium and substrate binding at the zwitterionic interface.
|
| |
Biochemistry,
35,
12164-12174.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.S.Chang,
P.F.Wu,
and
C.C.Chang
(1996).
cDNA sequence analysis and mutagenesis studies on the A chain of beta-bungarotoxin from Taiwan banded krait.
|
| |
J Protein Chem,
15,
755-761.
|
 |
|
|
|
|
 |
P.A.Cole
(1996).
Chaperone-assisted protein expression.
|
| |
Structure,
4,
239-242.
|
 |
|
|
|
|
 |
M.C.Tzeng,
C.H.Yen,
M.J.Hseu,
C.M.Dupureur,
and
M.D.Tsai
(1995).
Conversion of bovine pancreatic phospholipase A2 at a single site into a competitor of neurotoxic phospholipases A2 by site-directed mutagenesis.
|
| |
J Biol Chem,
270,
2120-2123.
|
 |
|
|
|
|
 |
R.Dua,
S.K.Wu,
and
W.Cho
(1995).
A structure-function study of bovine pancreatic phospholipase A2 using polymerized mixed liposomes.
|
| |
J Biol Chem,
270,
263-268.
|
 |
|
|
|
|
 |
S.H.Beiboer,
P.A.Franken,
R.C.Cox,
and
H.M.Verheij
(1995).
An extended binding pocket determines the polar head group specificity of porcine pancreatic phospholipase A2.
|
| |
Eur J Biochem,
231,
747-753.
|
 |
|
|
|
|
 |
A.Kumar,
C.Sekharudu,
B.Ramakrishnan,
C.M.Dupureur,
H.Zhu,
M.D.Tsai,
and
M.Sundaralingam
(1994).
Structure and function of the catalytic site mutant Asp 99 Asn of phospholipase A2: absence of the conserved structural water.
|
| |
Protein Sci,
3,
2082-2088.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.P.Ferreira,
R.Sasisekharan,
O.Louie,
and
R.Langer
(1994).
Carbodiimide modification enhances activity of pig pancreatic phospholipase A2.
|
| |
Eur J Biochem,
223,
611-616.
|
 |
|
|
|
|
 |
D.Hodgson,
S.Gasparini,
P.Drevet,
F.Ducancel,
F.Bouet,
J.C.Boulain,
J.B.Harris,
and
A.Menez
(1993).
Production of recombinant notechis 11'2L, an enzymatically active mutant of a phospholipase A2 from Notechis scutatus scutatus venom, as directly generated by cleavage of a fusion protein produced in Escherichia coli.
|
| |
Eur J Biochem,
212,
441-446.
|
 |
|
|
|
|
 |
R.B.Lugtigheid,
G.A.Nicolaes,
E.J.Veldhuizen,
A.J.Slotboom,
H.M.Verheij,
and
G.H.De Haas
(1993).
Acylation of porcine pancreatic phospholipase A2 influences penetration and substrate head-group binding, depending on the position of the acylated lysine in the enzyme molecule.
|
| |
Eur J Biochem,
216,
519-525.
|
 |
|
|
|
|
 |
C.Sekharudu,
B.Ramakrishnan,
B.Huang,
R.T.Jiang,
C.M.Dupureur,
M.D.Tsai,
and
M.Sundaralingam
(1992).
Crystal structure of the Y52F/Y73F double mutant of phospholipase A2: increased hydrophobic interactions of the phenyl groups compensate for the disrupted hydrogen bonds of the tyrosines.
|
| |
Protein Sci,
1,
1585-1594.
|
 |
|
|
|
|
 |
L.P.Vernon,
and
J.D.Bell
(1992).
Membrane structure, toxins and phospholipase A2 activity.
|
| |
Pharmacol Ther,
54,
269-295.
|
 |
|
|
|
|
 |
M.K.Jain,
B.Z.Yu,
M.H.Gelb,
and
O.G.Berg
(1992).
Assay of phospholipases A(2) and their inhibitors by kinetic analysis in the scooting mode.
|
| |
Mediators Inflamm,
1,
85.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

